459135
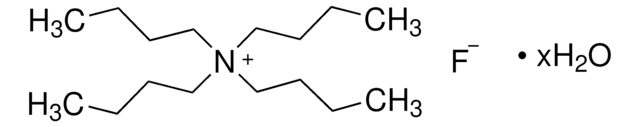
Tetramethylammonium fluoride
97%
Sinonimo/i:
TMAF
About This Item
Prodotti consigliati
Saggio
97%
Forma fisica
solid
Punto di fusione
170 °C (dec.) (lit.)
Stringa SMILE
[F-].C[N+](C)(C)C
InChI
1S/C4H12N.FH/c1-5(2,3)4;/h1-4H3;1H/q+1;/p-1
GTDKXDWWMOMSFL-UHFFFAOYSA-M
Categorie correlate
Applicazioni
- Halide-induced ring opening of 2-substituted aziridinium salts
- Synthesis of fluoro aromatics via fluorodenitration reactions
- Synthesis of sevoflurane in ionic liquids by halogen-exchange fluorination
- Synthesis of fluorinated Poly(oxomolybdates)
Avvertenze
Warning
Indicazioni di pericolo
Classi di pericolo
Acute Tox. 4 Dermal - Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Organi bersaglio
Respiratory system
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Dispositivi di protezione individuale
dust mask type N95 (US), Eyeshields, Gloves
Certificati d'analisi (COA)
Cerca il Certificati d'analisi (COA) digitando il numero di lotto/batch corrispondente. I numeri di lotto o di batch sono stampati sull'etichetta dei prodotti dopo la parola ‘Lotto’ o ‘Batch’.
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.