391743
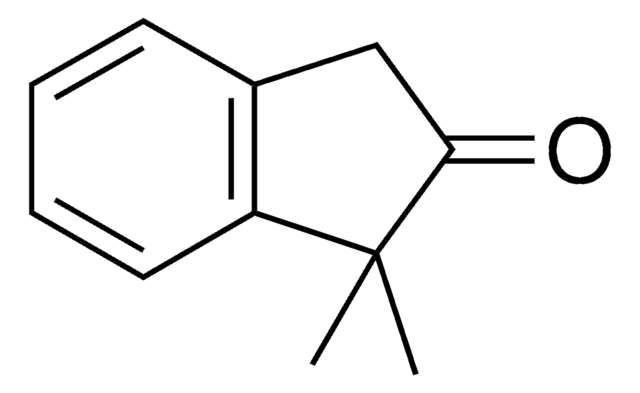
2-Methyl-1-indanone
99%
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
Formula empirica (notazione di Hill):
C10H10O
Numero CAS:
Peso molecolare:
146.19
Numero MDL:
Codice UNSPSC:
12352100
ID PubChem:
NACRES:
NA.22
Prodotti consigliati
Saggio
99%
Forma fisica
liquid
Indice di rifrazione
n20/D 1.555 (lit.)
P. eboll.
93-95 °C/4 mmHg (lit.)
Densità
1.064 g/mL at 25 °C (lit.)
Stringa SMILE
CC1Cc2ccccc2C1=O
InChI
1S/C10H10O/c1-7-6-8-4-2-3-5-9(8)10(7)11/h2-5,7H,6H2,1H3
BEKNOGMQVKBMQN-UHFFFAOYSA-N
Descrizione generale
2-Methyl-1-indanone, a α-benzocycloalkenone, is a derivative of 1-indanone. Its synthesis has been reported. The enzymatic dynamic kinetic resolution (DKR) of racemic 2-methyl-1-indanone has been studied. The asymmetric α-arylation and hydroxymethylation of 2-methyl-1-indanone has been reported. It participated in the synthesis of 2-methyl-6-carboxyazulene.
Applicazioni
2-Methyl-1-indanone may be used as a starting material in the synthesis of β-benzocycloalkenone. It may be used in the synthesis of the following:
- cyclohex-2-en-1-yl 2-methyl-1H-inden-3-yl carbonate
- 2-hydroxy-2-methyl-1-indanone
- O-alkoxycarbonylation of lithium enolates
Codice della classe di stoccaggio
10 - Combustible liquids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
Eyeshields, Gloves
Certificati d'analisi (COA)
Cerca il Certificati d'analisi (COA) digitando il numero di lotto/batch corrispondente. I numeri di lotto o di batch sono stampati sull'etichetta dei prodotti dopo la parola ‘Lotto’ o ‘Batch’.
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
Michał Rachwalski et al.
Chemical Society reviews, 42(24), 9268-9282 (2013-09-26)
Deracemisation of racemic compounds is still the most important strategy to produce optically pure compounds despite many recent advances in asymmetric synthesis. Especially deracemisation approaches that give rise to single enantiomers are preferred, which can be achieved either by invoking
Shaozhong Ge et al.
Journal of the American Chemical Society, 133(41), 16330-16333 (2011-09-16)
We report the α-arylation of ketones with a range of aryl chlorides with enantioselectivities from 90 to 99% ee catalyzed by the combination of Ni(COD)(2) and (R)-BINAP and the coupling of ketones with a range of heteroaryl chlorides with enantioselectivities
Michael W Justik et al.
Molecules (Basel, Switzerland), 10(1), 217-225 (2007-11-17)
The conversion of alpha-benzocycloalkenones to homologous beta-benzocyclo-alkenones containing six, seven and eight-membered rings is reported. This was accomplished via a Wittig olefination-oxidative rearrangement sequence using[hydroxy(tosyloxy)iodo]-benzene (HTIB) is the oxidant, that enables the synthesis of regioisomeric pairs of methyl-substituted beta-benzocycloalkenones. The
Cyclophanes. 9. anti-[2.2](2, 6) Azulenophane. Synthesis and charge-transfer interaction.
Luhowy R and Keehn PM.
Journal of the American Chemical Society, 99(11), 3797-3805 (1977)
On the decarboxylation of 2-methyl-1-tetralone-2-carboxylic acid-oxidation of the enol intermediate by triplet oxygen.
Riahi A, et al.
New. J. Chem., 37(8), 2245-2249 (2013)
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.