178594
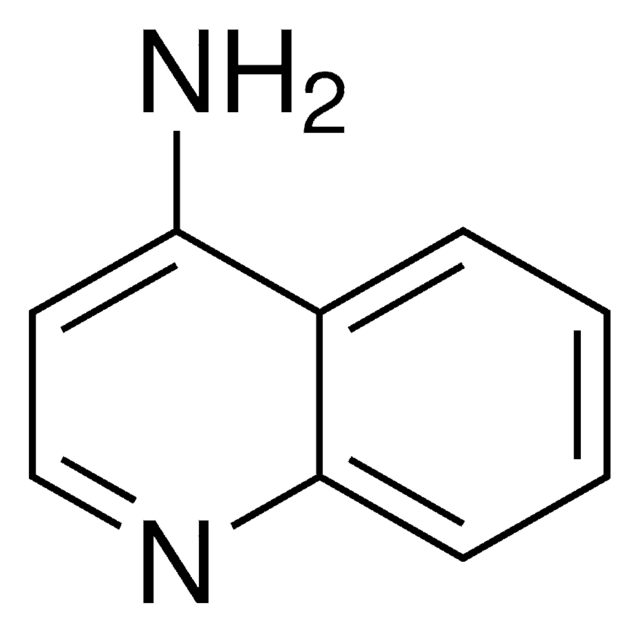
1-Aminoisoquinoline
99%
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
Formula empirica (notazione di Hill):
C9H8N2
Numero CAS:
Peso molecolare:
144.17
Numero CE:
Numero MDL:
Codice UNSPSC:
12352100
ID PubChem:
NACRES:
NA.22
Prodotti consigliati
Saggio
99%
Punto di fusione
122-124 °C (lit.)
Stringa SMILE
Nc1nccc2ccccc12
InChI
1S/C9H8N2/c10-9-8-4-2-1-3-7(8)5-6-11-9/h1-6H,(H2,10,11)
OSILBMSORKFRTB-UHFFFAOYSA-N
Applicazioni
1-Aminoisoquinoline was used in the synthesis of pyrimidoisoquinolinone.
Avvertenze
Warning
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Organi bersaglio
Respiratory system
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Dispositivi di protezione individuale
dust mask type N95 (US), Eyeshields, Gloves
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
Adrian L Smith et al.
Journal of medicinal chemistry, 52(20), 6189-6192 (2009-09-22)
The discovery and optimization of a novel series of aminoisoquinolines as potent, selective, and efficacious inhibitors of the mutant B-Raf pathway is presented. The N-linked pyridylpyrimidine benzamide 2 was identified as a potent, modestly selective inhibitor of the B-Raf enzyme.
Xiaohong Wei et al.
Organic letters, 13(17), 4636-4639 (2011-08-05)
[RhCp*Cl(2)](2) can catalyze the oxidative coupling of N-aryl and N-alkyl benzamidines with alkynes to give N-substituted 1-aminoisoquinolines in high selectivity.
[A practical method for the synthesis of 1-aminoisoquinoline].
S Giorgi-Renault et al.
Annales pharmaceutiques francaises, 41(6), 555-557 (1983-01-01)
J B Rewinkel et al.
Bioorganic & medicinal chemistry letters, 9(5), 685-690 (1999-04-14)
Replacement of the highly basic benzamidine moiety of NAPAP by the moderately basic 1-aminoisoquinoline moiety resulted in thrombin inhibitors with improved selectivity towards trypsin and enhanced Caco-2 cell permeability.
Hervé Bibas et al.
The Journal of organic chemistry, 67(8), 2619-2631 (2002-04-13)
The synthesis, spectroscopic properties, and chemical reactions of the stable (neopentylimino)-, (mesitylimino)-, and (o-tert-butylphenylimino)propadienones (6) are reported. Nucleophilic addition of amines affords the malonic amidoamidines 7 and 8. 3,5-Dimethylpyrazole reacts analogously to form 9b. Addition of 1,2-dimethylhydrazine produces pyrazolinones 10-12.
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.