38370
DIC
≥98.0% (GC), for peptide synthesis
Sinónimos:
N,N′-Diisopropilcarbodiimida
About This Item
Productos recomendados
product name
DIC, purum, ≥98.0% (GC)
grade
purum
Quality Level
assay
≥98.0% (GC)
form
liquid
reaction suitability
reaction type: Coupling Reactions
refractive index
n20/D 1.433 (lit.)
bp
145-148 °C (lit.)
density
0.815 g/mL at 20 °C (lit.)
0.815 g/mL at 20 °C
application(s)
peptide synthesis
functional group
amine
SMILES string
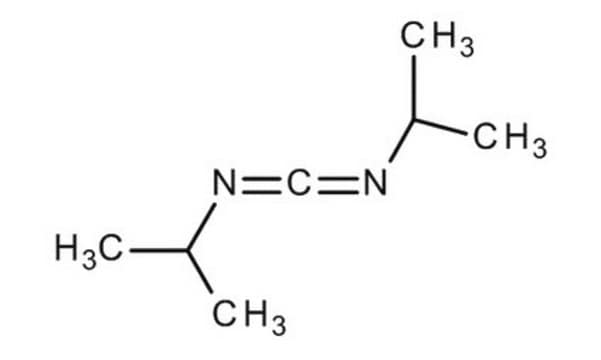
CC(C)N=C=NC(C)C
InChI
1S/C7H14N2/c1-6(2)8-5-9-7(3)4/h6-7H,1-4H3
Inchi Key
BDNKZNFMNDZQMI-UHFFFAOYSA-N
Gene Information
human ... EPHX2(2053)
mouse ... Ephx2(13850)
¿Está buscando productos similares? Visita Guía de comparación de productos
Categorías relacionadas
General description
Application
- To synthesize lanthanide (Ln) guanidinate complexes via insertion of carbodiimide into the Ln-N bond of lanthanocene secondary amido complexes.
- To facilitate the cyclization of N-(β-Hydroxy)amides to form 2-oxazolines.
- To synthesize 1-isopropyl-2-alkoxycarbonyl-3-isopropyliminio-aziridine by reacting with alkyl diazoacetates in the presence of transition metal salts.
- A coupling reagent for the synthesis of various esters and amides by treating carboxylic acids with phenols and amines respectively.
- A reagent for the conversion of alcohols to aldehydes or ketones in the presence of DMSO via modified Moffatt-type oxidation reaction.
- A reagent to facilitates the preparation of alkyl halides from corresponding alcohols via the formation of o-alkylisourea.
Related product
signalword
Danger
hcodes
Hazard Classifications
Acute Tox. 1 Inhalation - Eye Dam. 1 - Flam. Liq. 3 - Resp. Sens. 1 - Skin Sens. 1
Storage Class
3 - Flammable liquids
wgk_germany
WGK 3
flash_point_f
91.4 °F
flash_point_c
33 °C
ppe
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico