8.51086
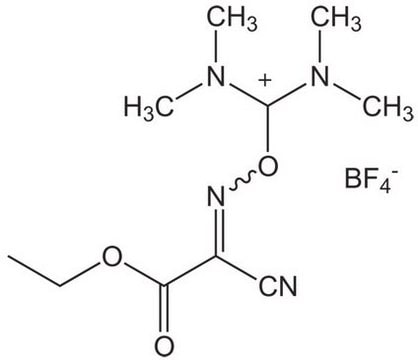
Oxyma Pure
≥99.0% (HPLC), for peptide synthesis, Novabiochem®
Sinónimos:
Oxyma Pure, Ethyl cyano(hydroxyimino)acetate
About This Item
Productos recomendados
product name
Oxyma Pure, Novabiochem®
Quality Level
product line
Novabiochem®
assay
≥99.0% (HPLC)
form
crystalline powder
reaction suitability
reaction type: Coupling Reactions
manufacturer/tradename
Novabiochem®
mp
130-132 °C
application(s)
peptide synthesis
storage temp.
2-8°C
InChI
1S/C5H6N2O3/c1-2-10-5(8)4(3-6)7-9/h9H,2H2,1H3/b7-4+
InChI key
LCFXLZAXGXOXAP-QPJJXVBHSA-N
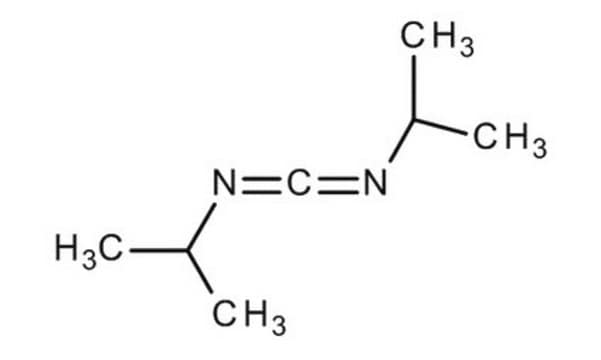
General description
The generation of toxic hydrogen cyanide (HCN) from the reaction between Oxyma Pure and DIC has been observed. Fortunately, this side reaction can be eliminated by substituting DIC for the more hindered t-butylethylcarbodiimide.
Associated Protocols and Technical Articles
Guide to Selection of Coupling Reagents
Literature references:
[1] R. Subirós-Funosas, et al. (2009) Chem. Eur. J., 15, 9394
[2] J. Collins , et al. (2014) Org . Lett ., 16, 940.
[3] A. D. McFarland (2019) Org. Process Res. Dev., 23, 2099.
[4] S. R. Manne, et al (2022) Org. Process Res. Dev., 26, 2894.
Application
- Use in the synthesis of difficult peptides.
- As an acidic modifier to prevent aspartimide formation.
Features and Benefits
- Can be used in place of HOBt in carbodiimide-mediated coupling reactions without change of protocol
- It gives results comparable to HOAt in step-wise solid-phase synthesis
- Less epimerization than HOBt in fragment condensation reactions
Analysis Note
Appearance of substance (visual): crystalline powder
Assay (HPLC, area%): ≥ 99.0 % (a/a)
Identity (IR): passes test
Solubility (12,5 mmol in 25 ml DMF): clearly soluble
Legal Information
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
Certificados de análisis (COA)
Busque Certificados de análisis (COA) introduciendo el número de lote del producto. Los números de lote se encuentran en la etiqueta del producto después de las palabras «Lot» o «Batch»
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico