73200
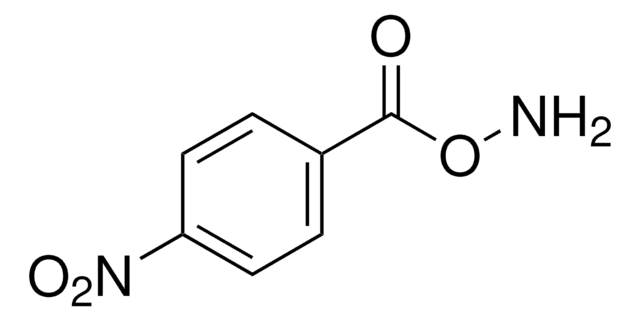
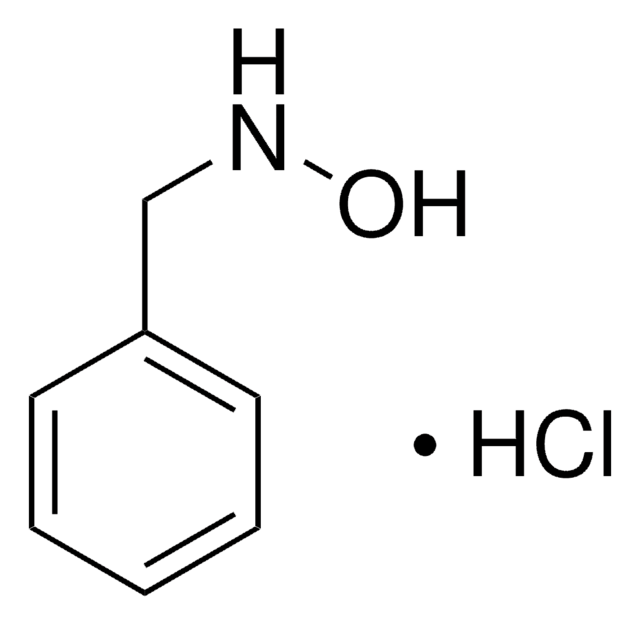
O-(4-Nitrobenzyl)hydroxylamine hydrochloride
≥98.5% (AT)
Sinónimos:
4-Nitrobenzyloxyamine hydrochloride
Iniciar sesiónpara Ver la Fijación de precios por contrato y de la organización
About This Item
Fórmula lineal:
O2NC6H4CH2ONH2 · HCl
Número de CAS:
Peso molecular:
204.61
Beilstein/REAXYS Number:
3709565
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Productos recomendados
Quality Level
assay
≥98.5% (AT)
form
crystals
mp
215 °C (dec.) (lit.)
functional group
nitro
SMILES string
Cl.NOCc1ccc(cc1)[N+]([O-])=O
InChI
1S/C7H8N2O3.ClH/c8-12-5-6-1-3-7(4-2-6)9(10)11;/h1-4H,5,8H2;1H
Inchi Key
LKCAFSOYOMFQSL-UHFFFAOYSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
Categorías relacionadas
Other Notes
Reagent for the preparation of N-(4-nitrobenzyloxy)-amino acids as substrates for an unambiguous N-hydroxypeptide synthesis
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, type N95 (US)
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Joo Hyun Lim et al.
Frontiers in microbiology, 9, 2333-2333 (2018-10-16)
2-Deoxy-scyllo-inosose (DOI) has been a valuable starting natural product for the manufacture of pharmaceuticals or chemical engineering resources such as pyranose catechol. DOI synthase, which uses glucose-6-phosphate (Glc6P) as a substrate for DOI biosynthesis, is indispensably involved in the initial
T D Traylor et al.
Journal of chromatography, 272(1), 9-20 (1983-01-14)
A new quantitative procedure for the high-performance liquid chromatographic (HPLC) resolution of human brain gangliosides employing reversed-phase chromatography is described. To provide a derivative which can be determined by UV absorption techniques, p-nitrobenzyloxyamine was coupled to the gangliosides. Derivatization involves
Kenneth P Roberts et al.
Chemical research in toxicology, 19(2), 300-309 (2006-02-21)
A new method has been developed to accurately measure apurinic and apyrimidinic (AP) DNA damage sites, which are lesions in DNA formed by loss of a nucleobase from oxidative stress or carcinogen adducts. If AP sites are left unrepaired (or
B X Chen et al.
Mutation research, 273(3), 253-261 (1992-05-01)
The abasic site is one of the most frequent changes occurring in DNA and has been shown to be lethal and mutagenic. An abasic site in DNA can be tagged by reaction with O-4-nitrobenzylhydroxylamine (NBHA), resulting in the formation of
M Pauly et al.
Carbohydrate research, 282(1), 1-12 (1996-02-28)
An improved procedure has been developed for the rapid derivatization of oligosaccharides with UV-detectable p-nitrobenzylhydroxylamine (PNB). The improved conditions used result in quantitative derivatization of neutral oligosaccharides. Sialylated oligosaccharides can also be quantitatively PNB-derivatized without detectable desialylation. Of the oligosaccharides
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico