13454
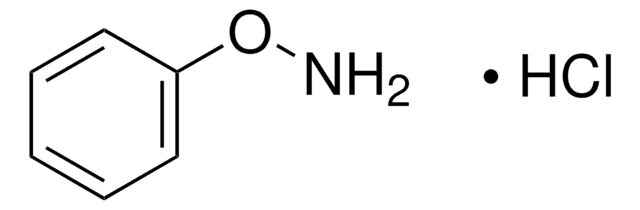
N-Benzylhydroxylamine hydrochloride
puriss., ≥99.0% (AT)
Iniciar sesiónpara Ver la Fijación de precios por contrato y de la organización
About This Item
Fórmula lineal:
C6H5CH2NHOH · HCl
Número de CAS:
Peso molecular:
159.61
Beilstein/REAXYS Number:
507948
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Productos recomendados
grade
puriss.
Quality Level
assay
≥99.0% (AT)
form
solid
mp
~105 °C
108-110 °C (lit.)
functional group
amine
phenyl
SMILES string
Cl.ONCc1ccccc1
InChI
1S/C7H9NO.ClH/c9-8-6-7-4-2-1-3-5-7;/h1-5,8-9H,6H2;1H
Inchi Key
YSNXOQGDHGUKCZ-UHFFFAOYSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
Categorías relacionadas
Application
N-Benzylhydroxylamine hydrochloride was used in the synthesis of sugar derived nitrones. It was used as starting reagent in the synthesis of fluoro isoxazoline and isoxazolidine derivatives using flouro nitrone.
Biochem/physiol Actions
N-Benzylhydroxylamine is a potential pharmacological agent in the prevention and progression of acrolein-induced damage to the retinal pigment epithelium.
Other Notes
Formation of nitrones from carbonyl compounds and [2+3]-cycloaddition with olefins to isoxazolidines, which are versatile intermediates; Pd-catalyzed hydroxylamination of allylic esters.
signalword
Warning
hcodes
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
target_organs
Respiratory system
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
dust mask type N95 (US), Eyeshields, Gloves
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
T. Kawakami et al.
Bulletin of the Chemical Society of Japan, 73, 2423-2423 (2000)
B.J. Wakefield
Sci. Synth., 11, 229-229 (2002)
Ionic liquid mediated synthesis of some novel fluoro isoxazolidine and isoxazoline derivatives using N-benzyl fluoro nitrone via cycloaddition reaction and their antimicrobial activities.
Chakraborty B, et al.
Indian J. Chem. B, 52(10), 1342-1351 (2013)
R Herrera et al.
The Journal of organic chemistry, 66(4), 1252-1263 (2001-04-21)
Captodative olefins 1-acetylvinyl carboxylates proved to be highly regioselective dipolarophiles in 1,3-dipolar cycloadditon to propionitrile oxide, arylphenylnitrile imines, diazoalkanes, and nitrones to yield the corresponding 5-substituted heterocycles. The addition of the latter was also stereoselective, being slightly susceptible to steric
J.E. Baldwin et al.
Tetrahedron, 42, 3097-3097 (1986)
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico