531197
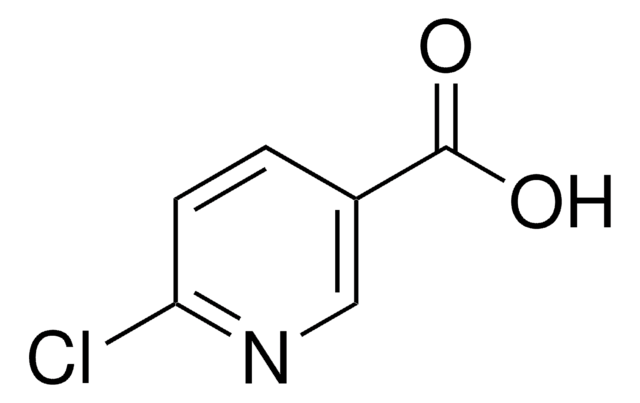
Ethyl 6-chloropyridine-3-carboxylate
97%
Sinónimos:
Ethyl 6-chloronicotinate
Iniciar sesiónpara Ver la Fijación de precios por contrato y de la organización
About This Item
Fórmula empírica (notación de Hill):
C8H8ClNO2
Número de CAS:
Peso molecular:
185.61
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Productos recomendados
assay
97%
mp
26-30 °C (lit.)
functional group
chloro
ester
SMILES string
CCOC(=O)c1ccc(Cl)nc1
InChI
1S/C8H8ClNO2/c1-2-12-8(11)6-3-4-7(9)10-5-6/h3-5H,2H2,1H3
InChI key
ILDJJTQWIZLGPO-UHFFFAOYSA-N
General description
Ethyl 6-chloropyridine-3-carboxylate (Ethyl-6-chloronicotinate, E-6-ClN) undergoes direct amidation on reacting with benzylamine in the presence lanthanum trifluoromethanesulfonate La(OTf)3. The structural and physicochemical properties of E-6-ClN have been investigated based on its spectroscopic data, time-dependent density functional theory and density of state diagrams.
Application
Ethyl 6-chloropyridine-3-carboxylate (Ethyl 6-chloronicotinate) may be used in the preparation of ethyl 5-{[5-(1H-benzimidazol-2-yl)pyridin-2-yl]ethynyl}pyridine-2-carboxylate by reacting with 2-[6-(ethynyl)pyridin-3-yl]-1H-benzimidazole under microwave irradiation.
signalword
Warning
hcodes
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
230.0 °F - closed cup
flash_point_c
110 °C - closed cup
ppe
dust mask type N95 (US), Eyeshields, Gloves
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Microwave-assisted Sonogashira coupling of novel 2-[6-(arylethynyl) pyridin-3-yl]-1H-benzimidazole derivatives.
Raut CN, et al.
ARKIVOC (Gainesville, FL, United States), 11, 105-114 (2009)
The spectroscopic (FT-IR, FT-Raman, dispersive Raman and NMR) study of ethyl-6-chloronicotinate molecule by combined density functional theory.
Karabacak M, et al.
Spectrochimica Acta. Part A, Molecular and Biomolecular Spectroscopy, 153, 754-770 (2016)
Hiroyuki Morimoto et al.
Organic letters, 16(7), 2018-2021 (2014-03-26)
Lanthanum trifluoromethanesulfonate is an effective single-component catalyst for synthesizing a variety of amides directly from esters and amines under mild conditions. Highly selective amidation of esters and amines, as well as catalyst-controlled amidation of esters, demonstrated the effectiveness of the
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico