15406
N-Boc-cadaverine
≥97.0% (NT)
Sinónimos:
N-Boc-1,5-diaminopentane, tert-Butyl N-(5-aminopentyl)carbamate
About This Item
Productos recomendados
Quality Level
assay
≥97.0% (NT)
reaction suitability
reagent type: cross-linking reagent
refractive index
n20/D 1.460
density
0.972 g/mL at 20 °C (lit.)
functional group
Boc
amine
SMILES string
NCCCCCNC(OC(C)(C)C)=O
InChI
1S/C10H22N2O2/c1-10(2,3)14-9(13)12-8-6-4-5-7-11/h4-8,11H2,1-3H3,(H,12,13)
InChI key
DPLOGSUBQDREOU-UHFFFAOYSA-N
Application
- Synthesis of of a supermacrocycle that self-assemble to form organic nanotubes.
- Preparation of water-soluble unsymmetrical sulforhodamine fluorophores from monobrominated sulfoxanthene dye.
- Synthesis of functionalized porphyrins as biocompatible carrier system for photodynamic therapy (PDT).
Other Notes
signalword
Danger
hcodes
Hazard Classifications
Skin Corr. 1B
Storage Class
8A - Combustible corrosive hazardous materials
wgk_germany
WGK 3
flash_point_f
228.2 °F - closed cup
flash_point_c
109.0 °C - closed cup
ppe
Faceshields, Gloves, Goggles, type ABEK (EN14387) respirator filter
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Artículos
Mono-Boc-protected diamines are versatile building blocks for chemical synthesis. Their production is a lot more challenging than the simple reaction scheme might imply, because the Boc-anhydride reagent cannot differentiate between the two identical amino moieties in the substrate.
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico










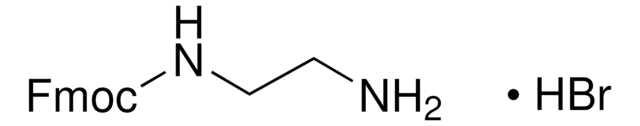

![2-[2-(Boc-amino)ethoxy]ethanol 97%](/deepweb/assets/sigmaaldrich/product/structures/413/416/884359e5-1cb4-4071-bb4f-28d9844db662/640/884359e5-1cb4-4071-bb4f-28d9844db662.png)
