15404
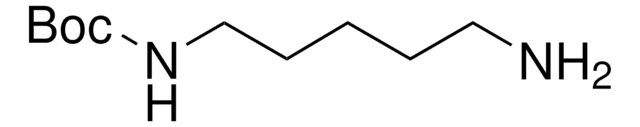
N-Boc-1,4-butanediamine
≥97.0% (GC/NT)
Sinónimos:
N-Boc-1,4-diaminobutane, tert-Butyl N-(4-aminobutyl)carbamate
About This Item
Productos recomendados
Quality Level
assay
≥97.0% (GC/NT)
reaction suitability
reagent type: cross-linking reagent
refractive index
n20/D 1.460
density
0.984 g/mL at 20 °C (lit.)
functional group
Boc
amine
SMILES string
NCCCCNC(OC(C)(C)C)=O
InChI
1S/C9H20N2O2/c1-9(2,3)13-8(12)11-7-5-4-6-10/h4-7,10H2,1-3H3,(H,11,12)
InChI key
ZFQWJXFJJZUVPI-UHFFFAOYSA-N
Categorías relacionadas
Application
- Carboxy-Silane Coated Iron Oxide Nanoparticles: Details the application of N-Boc-1,4-butanediamine in modifying iron oxide nanoparticles for imaging and drug delivery (D Stanicki, S Boutry, S Laurent, et al., 2014). Access the article.
Other Notes
signalword
Danger
hcodes
Hazard Classifications
Skin Corr. 1B
Storage Class
8A - Combustible corrosive hazardous materials
wgk_germany
WGK 3
flash_point_f
228.2 °F - closed cup
flash_point_c
109.0 °C - closed cup
ppe
Faceshields, Gloves, Goggles, type ABEK (EN14387) respirator filter
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Artículos
Mono-Boc-protected diamines are versatile building blocks for chemical synthesis. Their production is a lot more challenging than the simple reaction scheme might imply, because the Boc-anhydride reagent cannot differentiate between the two identical amino moieties in the substrate.
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico