1435003
USP
甲基强的松龙
United States Pharmacopeia (USP) Reference Standard
别名:
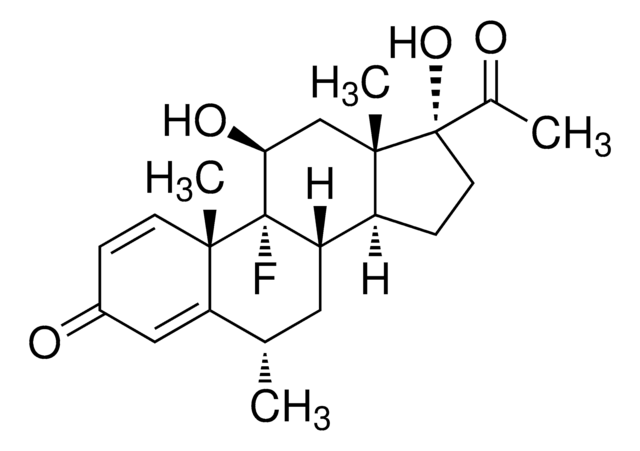
6α-甲基泼尼松龙, 11β,17α,21-三羟基-6α-甲基-1,4-孕甾二烯-3,20-二酮, 6α-甲基-11β,17α,21-三羟基-1,4-孕甾二烯-3,20-二酮, 甲基强的松龙, 甲基泼尼松龙
About This Item
推荐产品
等級
pharmaceutical primary standard
API 家族
methylprednisolone
製造商/商標名
USP
應用
pharmaceutical (small molecule)
格式
neat
SMILES 字串
[H][C@@]12C[C@H](C)C3=CC(=O)C=C[C@]3(C)[C@@]1([H])[C@@H](O)C[C@@]4(C)[C@@]2([H])CC[C@]4(O)C(=O)CO
InChI
1S/C22H30O5/c1-12-8-14-15-5-7-22(27,18(26)11-23)21(15,3)10-17(25)19(14)20(2)6-4-13(24)9-16(12)20/h4,6,9,12,14-15,17,19,23,25,27H,5,7-8,10-11H2,1-3H3/t12-,14-,15-,17-,19+,20-,21-,22-/m0/s1
InChI 密鑰
VHRSUDSXCMQTMA-PJHHCJLFSA-N
基因資訊
human ... NR3C1(2908)
正在寻找类似产品? 访问 产品对比指南
一般說明
應用
- Methylprednisolone Sodium Succinate for Injection
- Methylprednisolone Tablets
分析報告
其他說明
相關產品
訊號詞
Danger
危險聲明
危險分類
Repr. 1B - STOT RE 2
標靶器官
Adrenal gland,Immune system
儲存類別代碼
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
水污染物質分類(WGK)
WGK 2
其他客户在看
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门