所有图片(1)
About This Item
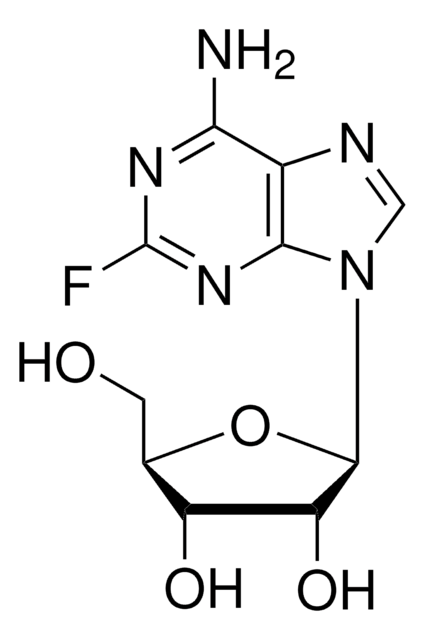
经验公式(希尔记法):
C15H22FN3O6
CAS号:
分子量:
359.35
MDL编号:
UNSPSC代码:
12352200
PubChem化学物质编号:
NACRES:
NA.77
推荐产品
质量水平
方案
≥98% (HPLC)
表单
powder
旋光性
[α]/D +80 to +100°, c = 0.5 in methanol
颜色
white to beige
溶解性
H2O: 10 mg/mL, clear (warmed)
储存温度
2-8°C
SMILES字符串
O[C@H]1[C@@H](O)[C@H](N2C(N=C(NC(OCCCCC)=O)C(F)=C2)=O)O[C@@H]1C
InChI
1S/C15H22FN3O6/c1-3-4-5-6-24-15(23)18-12-9(16)7-19(14(22)17-12)13-11(21)10(20)8(2)25-13/h7-8,10-11,13,20-21H,3-6H2,1-2H3,(H,17,18,22,23)/t8-,10-,11-,13-/m1/s1
InChI key
GAGWJHPBXLXJQN-UORFTKCHSA-N
基因信息
human ... TYMS(7298)
正在寻找类似产品? 访问 产品对比指南
应用
Capecitabine has been used:
- in combination with gemcitabine to achieve glutamine deprivation by enhancing the sensitivity of expression in pancreatic ductal adenocarcinoma (PDAC) cells to inhibitors of glutamine metabolism and study its effect on PDAC cell survival
- to study the drug metabolic function in a two-organ microfluidic system
- as an anti-cancer agent to study its cytotoxic activity alone or in combination with B87 on cancer cells
生化/生理作用
卡培他滨是一种抗癌药物,是去氧氟尿苷的前药,在肿瘤部位代谢为5-氟尿嘧啶。
卡培他滨是一种抗癌药物,是去氧氟尿苷的前药,在肿瘤部位代谢为5-氟尿嘧啶。 卡培他滨的激活遵循具有三个酶促步骤和两个中间代谢物5′-脱氧-5-氟胞苷(5′-DFCR)和5′-脱氧-5-氟尿苷(5′-DFUR)的途径,形成5-氟尿嘧啶。
警示用语:
Danger
危险声明
危险分类
Carc. 1B - Muta. 2 - Repr. 1B
储存分类代码
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 3
闪点(°F)
Not applicable
闪点(°C)
Not applicable
历史批次信息供参考:
分析证书(COA)
Lot/Batch Number
其他客户在看
Mitsuhiro Tomoda et al.
Anticancer research, 34(1), 191-194 (2014-01-10)
Unresectable metastatic colorectal cancer with very slow tumour growth rate does not necessarily require for strong short-interval chemotherapy. In the present study, we administered monthly chemotherapy and aimed to evaluate the usefulness of the specific treatment schedule in patients with
Capecitabine and streptozocin ± cisplatin in advanced gastroenteropancreatic neuroendocrine tumours.
Tim Meyer et al.
European journal of cancer (Oxford, England : 1990), 50(5), 902-911 (2014-01-22)
Cytotoxic chemotherapy is widely used for advanced, unresectable pancreatic and other gastrointestinal foregut neuroendocrine tumours (NETs) and the most commonly used regimen combines 5-fluorouracil with streptozocin. The NET01 trial was designed to investigate whether capecitabine combined with streptozocin was an
Francesco Giotta et al.
Tumori, 99(6), 278e-281e (2014-02-08)
We present the case of a 58-year-old woman with breast cancer metastasizing to the liver after adjuvant chemotherapy. A liver biopsy confirmed metastatic lesions from breast cancer that were immunohistochemically positive for estrogen/progesterone receptors and HER2. After first-line treatment with
Dermatomyositis associated with capecitabine in the setting of malignancy.
Frank W Chen et al.
Journal of the American Academy of Dermatology, 70(2), e47-e48 (2014-01-21)
Karen-Lise G Spindler et al.
Anticancer research, 34(2), 845-850 (2014-02-11)
We investigated the efficacy and safety of capecitabine and gemcitabin (GemCap) in heavily pre-treated, therapy-resistant metastatic colorectal cancer (mCRC) patients and the clinical importance of cell-free DNA (cfDNA) measurement. Patients' inclusion criteria included histopathologically-verified mCRC refractory to standard chemotherapy, adequate
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系客户支持