推荐产品
生物源
bovine bile
synthetic
化驗
≥95%
分子量
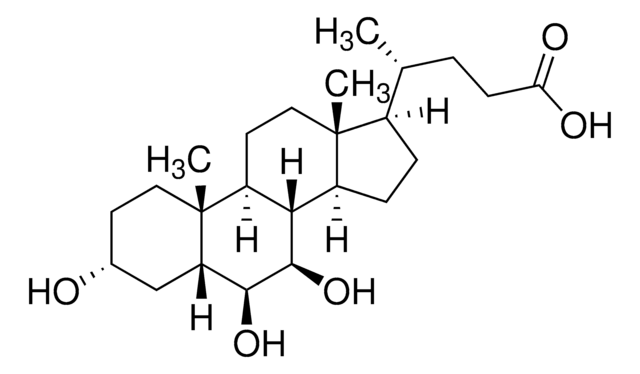
376.57 g/mol
mp
183-188 °C (lit.)
官能基
carboxylic acid
運輸包裝
ambient
儲存溫度
room temp
SMILES 字串
[H][C@]12CC[C@@]3([H])[C@]4([H])CC[C@H]([C@H](C)CCC(O)=O)[C@@]4(C)CC[C@]3([H])[C@@]1(C)CC[C@@H](O)C2
InChI
1S/C24H40O3/c1-15(4-9-22(26)27)19-7-8-20-18-6-5-16-14-17(25)10-12-23(16,2)21(18)11-13-24(19,20)3/h15-21,25H,4-14H2,1-3H3,(H,26,27)/t15-,16-,17-,18+,19-,20+,21+,23+,24-/m1/s1
InChI 密鑰
SMEROWZSTRWXGI-HVATVPOCSA-N
基因資訊
human ... POLA1(5422) , TOP2A(7153)
rat ... Polb(29240)
正在寻找类似产品? 访问 产品对比指南
一般說明
應用
生化/生理作用
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 2
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
Eyeshields, Gloves, type N95 (US)
其他客户在看
实验方案
Investigate bile acid roles in gut hormone profiles and glycemic control, vital for clinical labs exploring potential mechanisms.
相关内容
Bile Acids (BA) are synthesized in the liver and play important roles in cholesterol homeostasis, absorption of vitamins and lipids, and various key metabolic processes.
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门