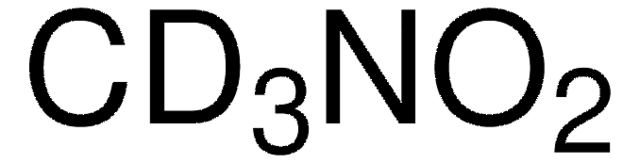推荐产品
蒸汽密度
2.1 (vs air)
蒸汽壓力
2.7 mmHg
產品線
ReagentPlus®
化驗
≥99.0%
形狀
liquid
自燃溫度
784 °F
expl. lim.
7.3 %, 33 °F
折射率
n20/D 1.382 (lit.)
pH值
6.4 (20 °C, 0.01 g/L)
bp
101.2 °C (lit.)
mp
−29 °C (lit.)
密度
1.127 g/mL at 25 °C (lit.)
SMILES 字串
C[N+]([O-])=O
InChI
1S/CH3NO2/c1-2(3)4/h1H3
InChI 密鑰
LYGJENNIWJXYER-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
一般說明
Nitromethane (CH3NO2) is the simplest nitro organic compound used for a wide range of applications, including as polar solvents to racing fuel. It serves as a solvent for organic chemistry and as a valuable building block for various useful compounds like nitroalkanes, β-nitroalcohols, nitroalkenes, carbonyl compounds, amines, and heterocycles. In industry, nitromethane can be used to stabilize halogenated hydrocarbons.
應用
Nitromethane can be used:
- As a reagent in the synthesis of 3-nitroindoles by arylation with N-(2-bromoaryl) imidates using palladium catalyst.
- As a cyanating reagent for the synthesis of thiocyanates in presence of base (KOAc) and iodine.
- In the preparation of cobalt cage complexes from polyamines and formaldehyde.
- As a solvent in the preparation of β-substituted γ-nitroaldehydes by enantioselective cross-coupling of β-arylated or γ,δ-unsaturated aldehydes using oxidizing agent DDQ (2,3-dichloro-5,6-dicyanoquinone) and catalyst diphenylprolinol silyl ether.
特點和優勢
Nitromethane as high energy monopropellant exhibits
- Low toxicity.
- High specific impulse.
法律資訊
ReagentPlus is a registered trademark of Merck KGaA, Darmstadt, Germany
訊號詞
Warning
危險分類
Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Carc. 2 - Flam. Liq. 3 - Repr. 2
儲存類別代碼
4.1A - Other explosive hazardous materials
水污染物質分類(WGK)
WGK 2
閃點(°F)
95.0 °F - closed cup
閃點(°C)
35 °C - closed cup
其他客户在看
The catalytic chemistry of nitromethane over Co-ZSM5 and other catalysts in connection with the methane-NOxSCR reaction.
Cowan AD, et al.
J. Catal., 176(2), 329-343 (1998)
Metal ion encapsulation: cobalt cages derived from polyamines, formaldehyde, and nitromethane.
Geue RJ, et al.
Journal of the American Chemical Society, 106(19), 5478-5488 (1984)
Benedek Vakulya et al.
Organic letters, 7(10), 1967-1969 (2005-05-07)
Cinchona alkaloid-derived chiral bifunctional thiourea organocatalysts were synthesized and applied in the Michael addition between nitromethane and chalcones with high ee and chemical yields.
Wenguo Yang et al.
Chemistry, an Asian journal, 7(4), 771-777 (2012-02-10)
Through the cleavage of the C-C bond, the first catalytic tandem conjugate addition-elimination reaction of Morita-Baylis-Hillman C adducts has been presented. Various S(N)2'-like C-, S-, and P-allylic compounds could be obtained with exclusive E configuration in good to excellent yields.
Eagleson M.
Concise Encyclopedia Chemistry, 696-696 (1994)
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门