所有图片(1)
About This Item
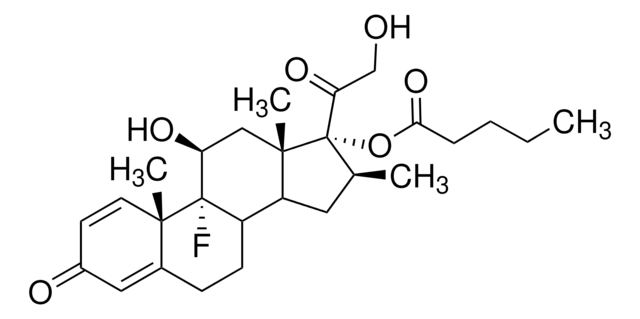
经验公式(希尔记法):
C31H48O6
CAS号:
分子量:
516.71
MDL编号:
UNSPSC代码:
41116107
PubChem化学物质编号:
NACRES:
NA.24
推荐产品
等级
pharmaceutical primary standard
API类
fusidic acid
制造商/商品名称
EDQM
应用
pharmaceutical (small molecule)
包装形式
neat
储存温度
−20°C
SMILES字符串
[H][C@@]12CC[C@@]3(C)[C@@]([H])([C@H](O)C[C@@]4([H])\C([C@H](C[C@]34C)OC(C)=O)=C(/CC\C=C(\C)C)C(O)=O)[C@@]1(C)CC[C@@H](O)[C@H]2C
InChI
1S/C31H48O6/c1-17(2)9-8-10-20(28(35)36)26-22-15-24(34)27-29(5)13-12-23(33)18(3)21(29)11-14-30(27,6)31(22,7)16-25(26)37-19(4)32/h9,18,21-25,27,33-34H,8,10-16H2,1-7H3,(H,35,36)/b26-20-/t18-,21-,22-,23+,24+,25-,27-,29-,30-,31-/m0/s1
InChI key
IECPWNUMDGFDKC-MZJAQBGESA-N
正在寻找类似产品? 访问 产品对比指南
一般描述
This product is provided as delivered and specified by the issuing Pharmacopoeia. All information provided in support of this product, including SDS and any product information leaflets have been developed and issued under the Authority of the issuing Pharmacopoeia.For further information and support please go to the website of the issuing Pharmacopoeia.
应用
Fusidic acid EP Reference standard, intended for use in laboratory tests only as specifically prescribed in the European Pharmacopoeia.
生化/生理作用
Suppresses nitric oxide lysis of pancreatic islet cells. Inhibits protein synthesis in prokaryotes by inhibiting the ribosome-dependent activity of G factor and translocation of peptidyl-tRNA.
Suppresses nitric oxide lysis of pancreatic islet cells; inhibits protein synthesis in prokaryotes.
包装
The product is delivered as supplied by the issuing Pharmacopoeia. For the current unit quantity, please visit the EDQM reference substance catalogue.
其他说明
Sales restrictions may apply.
警示用语:
Warning
危险声明
预防措施声明
危险分类
Acute Tox. 4 Oral
储存分类代码
11 - Combustible Solids
WGK
WGK 3
闪点(°F)
Not applicable
闪点(°C)
Not applicable
其他客户在看
Benjamin P Howden et al.
Clinical infectious diseases : an official publication of the Infectious Diseases Society of America, 42(3), 394-400 (2006-01-05)
Fusidic acid has activity against a range of pathogens but has mainly been used to treat staphylococcal infections. Fusidic acid monotherapy, especially topical preparations, has been strongly associated with the emergence of fusidic acid resistance among both methicillin-resistant Staphylococcus aureus
K Christiansen
International journal of antimicrobial agents, 12 Suppl 2, S3-S9 (1999-10-21)
Unlike trials conducted today on new antimicrobials, the introduction of fusidic acid was not accompanied by extensive studies on toxicity and side effects. The early studies on small numbers of patients reported fusidic acid to be a nontoxic drug with
J D Wilkinson
The British journal of dermatology, 139 Suppl 53, 37-40 (1999-02-17)
Fusidic acid is an antibiotic that belongs to a group of its own, the fusidanes. The molecule has a steroid-like structure but does not possess any steroid activity. The structure is thought to be responsible for the steroid-like high penetration
J Turnidge et al.
International journal of antimicrobial agents, 12 Suppl 2, S35-S44 (1999-10-21)
Resistance to fusidic acid is determined by a number of mechanisms. The best described are alterations in elongation factor G, which appear in natural mutants that are harboured at low rates in normal populations of staphylococci (10(6) to 10(8)). Altered
J Turnidge
International journal of antimicrobial agents, 12 Suppl 2, S23-S34 (1999-10-21)
Fusidic acid comes in a variety of formulations for oral, intravenous and topical use. After oral administration of 500 mg Cmax values range from 14.5-3.3 mg/l and an elimination half-life of 8.9-11.0 h. Similar values are obtained with intravenous administration
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系客户支持