Y0000582
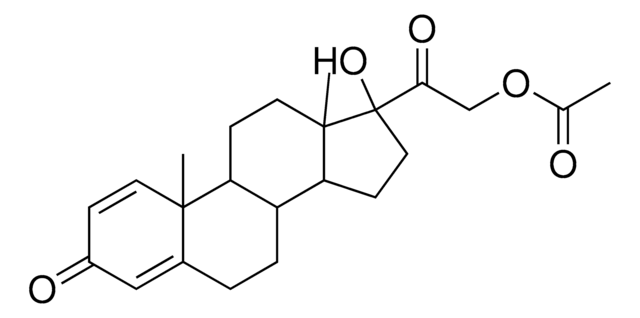
峰鉴别用醋酸泼尼松龙
European Pharmacopoeia (EP) Reference Standard
别名:
醋酸泼尼松龙, 1,4-孕甾二烯-11β,17α,21-三醇-3,20-二酮 21-醋酸酯, 11β,17α,21-三羟基-1,4-孕甾二烯-3,20-二酮 21-醋酸酯, 21-乙酰氧基-1,4-孕甾二烯-11β,17α-二醇-3,20-二酮
登录查看公司和协议定价
所有图片(1)
About This Item
经验公式(希尔记法):
C23H30O6
CAS号:
分子量:
402.48
Beilstein:
3111798
MDL號碼:
分類程式碼代碼:
41116107
PubChem物質ID:
NACRES:
NA.24
推荐产品
等級
pharmaceutical primary standard
API 家族
prednisolone
製造商/商標名
EDQM
應用
pharmaceutical (small molecule)
格式
neat
儲存溫度
2-8°C
SMILES 字串
[H][C@@]12CCC3=CC(=O)C=C[C@]3(C)[C@@]1([H])[C@@H](O)C[C@@]4(C)[C@@]2([H])CC[C@]4(O)C(=O)COC(C)=O
InChI
1S/C23H30O6/c1-13(24)29-12-19(27)23(28)9-7-17-16-5-4-14-10-15(25)6-8-21(14,2)20(16)18(26)11-22(17,23)3/h6,8,10,16-18,20,26,28H,4-5,7,9,11-12H2,1-3H3/t16-,17-,18-,20+,21-,22-,23-/m0/s1
InChI 密鑰
LRJOMUJRLNCICJ-JZYPGELDSA-N
基因資訊
human ... NR3C1(2908)
正在寻找类似产品? 访问 产品对比指南
一般說明
This product is provided as delivered and specified by the issuing Pharmacopoeia. All information provided in support of this product, including SDS and any product information leaflets have been developed and issued under the Authority of the issuing Pharmacopoeia.For further information and support please go to the website of the issuing Pharmacopoeia.
應用
Prednisolone acetate for peak identification EP Reference standard, intended for use in laboratory tests only as specifically prescribed in the European Pharmacopoeia.
包裝
The product is delivered as supplied by the issuing Pharmacopoeia. For the current unit quantity, please visit the EDQM reference substance catalogue.
其他說明
Sales restrictions may apply.
相關產品
产品编号
说明
价格
訊號詞
Danger
危險聲明
危險分類
Repr. 1B
儲存類別代碼
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
水污染物質分類(WGK)
WGK 3
Marcus Ang et al.
American journal of ophthalmology, 157(6), 1163-1169 (2014-03-04)
To compare 3-year endothelial cell loss and graft survival following Descemet stripping automated endothelial keratoplasty (DSAEK) using the EndoGlide (AngioTech, Reading, Pennsylvania, USA/Network Medical Products, North Yorkshire, UK) donor insertion device compared to donor insertion using the Sheets glide technique.
Salima I Hassanaly et al.
American journal of ophthalmology, 158(2), 270-276 (2014-05-23)
To describe outcomes after Boston Type 1 Keratoprosthesis (KPro) surgery in aniridic eyes. Retrospective, interventional case series. University-based tertiary care institution. Twenty-six aniridic eyes of 19 patients who underwent KPro implantation by a single experienced surgeon (M.H.-D.) between October 27
Oscar A Candia et al.
Experimental eye research, 128, 114-116 (2014-10-12)
We have previously shown that tissue plasminogen activator (tPA) injected in the vitreous of sheep, reduced or prevented the elevation of the intraocular pressure (IOP) normally produced by the instillation of 1% prednisolone. We now report the effect of tPA
Rohit Shetty et al.
American journal of ophthalmology, 159(3), 419-425 (2014-12-03)
To evaluate the effect of keratoconus cone location on the change in refractive outcomes, corneal aberrations, and biomechanics after combined topography-guided photorefractive keratectomy (PRK) and collagen cross-linking (CXL). Prospective, comparative case series. Topography-guided PRK was performed followed by accelerated CXL
Rohit Shetty et al.
Indian journal of ophthalmology, 62(9), 923-926 (2014-11-06)
To study the safety and efficacy of sutureless femtosecond anterior lamellar keratoplasty (FALK) in patients with corneal stromal opacities. Eleven eyes of 11 consecutive patients with corneal stromal opacities involving < 250 μ due to various pathologies were included in
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门