推荐产品
等級
certified reference material
pharmaceutical secondary standard
品質等級
agency
traceable to Ph. Eur. Y0000654
traceable to USP 1356960
API 家族
leflunomide
CofA
current certificate can be downloaded
技術
HPLC: suitable
gas chromatography (GC): suitable
應用
pharmaceutical (small molecule)
格式
neat
儲存溫度
2-8°C
SMILES 字串
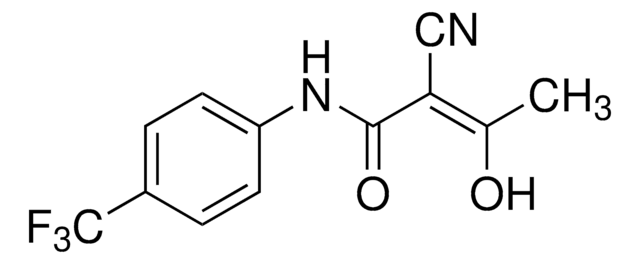
Cc1oncc1C(=O)Nc2ccc(cc2)C(F)(F)F
InChI
1S/C12H9F3N2O2/c1-7-10(6-16-19-7)11(18)17-9-4-2-8(3-5-9)12(13,14)15/h2-6H,1H3,(H,17,18)
InChI 密鑰
VHOGYURTWQBHIL-UHFFFAOYSA-N
基因資訊
human ... DHODH(1723)
正在寻找类似产品? 访问 产品对比指南
一般說明
Pharmaceutical secondary standards for application in quality control, provide pharma laboratories and manufacturers with a convenient and cost-effective alternative to the preparation of in-house working standards. Leflunomide belongs to the new class of immunomodulating drugs with inflammatory and immunomodulating properties. It has been investigated for application in transplantation procedures. It is also an anti-rheumatic drug (DMARD) used to treat rheumatoid arthritis (RA). Its mode of action involves the inhibition of T-cell proliferation after converting to its active metabolite i.e. A771726 in humans.
應用
Leflunomide may be used as a pharmaceutical reference standard for the quantification of the analyte in pharmaceutical formulations using chromatography techniques.
These Secondary Standards are qualified as Certified Reference Materials. These are suitable for use in several analytical applications including but not limited to pharma release testing, pharma method development for qualitative and quantitative analyses, food and beverage quality control testing, and other calibration requirements.
分析報告
These secondary standards offer multi-traceability to the USP, EP (PhEur) and BP primary standards, where they are available.
其他說明
This Certified Reference Material (CRM) is produced and certified in accordance with ISO 17034 and ISO/IEC 17025. All information regarding the use of this CRM can be found on the certificate of analysis.
腳註
To see an example of a Certificate of Analysis for this material enter LRAA1634 in the slot below. This is an example certificate only and may not be the lot that you receive.
相關產品
产品编号
说明
价格
訊號詞
Danger
危險分類
Acute Tox. 3 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
水污染物質分類(WGK)
WGK 1
閃點(°F)
Not applicable
閃點(°C)
Not applicable
其他客户在看
Development and Validation of a liquid chromatographic method for the determination of leflunomide: application to in vitro drug metal interactions
Sultana N, et al.
Chin. J. Chem., 29(9), 1933-1938 (2011)
Development of liquid chromatography?UV method for simultaneous determination of leflunomide and NSAIDS in API and pharmaceutical formulations: its application to in vitro interaction studies
Sultana N, et al.
Medicinal Chemistry, 3, 262-270 (2013)
Helen I Keen et al.
Expert opinion on drug safety, 12(4), 581-588 (2013-05-15)
Leflunomide is a prodrug which is rapidly converted following oral administration and absorption to an active metabolite with anti-proliferative effects (A77 1726/teriflunomide). Leflunomide was developed as an immunomodulatory agent and subsequently developed as a disease-modifying anti-rheumatic drug (DMARD) for the
Young H Jung et al.
Pediatric transplantation, 17(2), E50-E54 (2012-12-06)
The BK virus (BKV) can be reactivated with immunosuppressive treatment in renal allograft recipients, which can result in interstitial nephritis (BKV-associated nephropathy, BKVAN) and lead to renal allograft failure. Recently, leflunomide has been reported in some case series of BKVAN
Dominik Golicki et al.
Polskie Archiwum Medycyny Wewnetrznej, 122(1-2), 22-32 (2012-02-23)
Rheumatoid arthritis (RA) is a chronic systemic disease of the connective tissue that leads to progressive joint destruction, disability, withdrawal from occupational activity, and premature death. The aim of the paper was to evaluate the efficacy and safety of leflunomide
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门