推荐产品
一般說明
A set of eight ready to use cell-permeable, irreversible inhibitors of various caspase-family proteases. Contains 25 µl (2 mM) each of Caspase-1 Inhibitor VI, Z-YVAD-FMK (Cat. No. 218746); Caspase-2 Inhibitor I, Z-VDVAD-FMK (Cat. No. 218744); Caspase-3 Inhibitor II, Z-DEVD-FMK (Cat. No. 264155); Caspase-5 Inhibitor I, Z-WEHD-FMK (Cat. No. 218753); Caspase-6 Inhibitor I, Z-VEID-FMK (Cat. No. 218757); Caspase-8 Inhibitor II, Z-IETD-FMK (Cat. No. 218759); Caspase-9 Inhibitor I, Z-LEHD-FMK (Cat. No. 218761); and Caspase Inhibitor I, Z-VAD-FMK (Cat. No. 627610). Supplied with a data sheet.
Apoptosis is a normal process in development and morphogenesis. Many cells can be activated to undergo apoptosis following the interaction of selected ligands with cell surface receptors. Receptor-mediated apoptosis involves the activation of caspases (cysteine-containing aspartate-specific proteases). A distinctive feature of caspases is the requirement of an aspartic acid residue in the substrate P1 position.
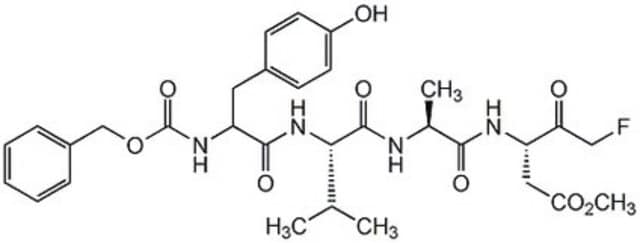
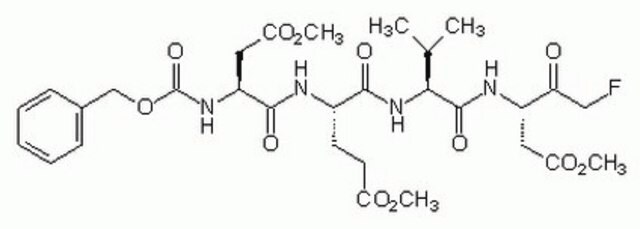
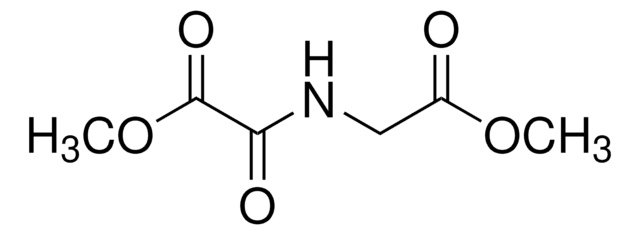
Caspase inhibitors act by binding to the active site of caspases and form either a reversible or an irreversible linkage. Caspase inhibitor design includes a peptide recognition sequence attached to a functional group such as an aldehyde (CHO), chloromethylketone (CMK), fluoromethylketone (FMK), or fluoroacyloxymethylketone (FAOM). Caspase inhibitors with a CHO group are reversible and those with a CMK, FMK, or FAOM group are irreversible and cell-permeable. FMK exhibits slightly less reactivity than CMK and therefore is more specific for the enzyme being inhibited.
Caspase inhibitors act by binding to the active site of caspases and form either a reversible or an irreversible linkage. Caspase inhibitor design includes a peptide recognition sequence attached to a functional group such as an aldehyde (CHO), chloromethylketone (CMK), fluoromethylketone (FMK), or fluoroacyloxymethylketone (FAOM). Caspase inhibitors with a CHO group are reversible and those with a CMK, FMK, or FAOM group are irreversible and cell-permeable. FMK exhibits slightly less reactivity than CMK and therefore is more specific for the enzyme being inhibited.
生化/生理作用
Cell permeable: yes
Primary Target
Caspase-1, caspase-2, caspase-3, caspase-4, caspase-5, caspase-6, caspase-7, caspase-8
Caspase-1, caspase-2, caspase-3, caspase-4, caspase-5, caspase-6, caspase-7, caspase-8
Reversible: no
警告
Toxicity: Irritant (B)
外觀
Supplied as 2 mM in DMSO.
其他說明
Humke, E.W., et al. 1998. J. Biol. Chem.273, 15702.
Datta, R., et al. 1997. J. Biol. Chem. 272, 1965.
Martin, L.M., et al. 1997. J. Biol. Chem. 272, 7421.
Takahashi, A., et al. 1997. Exp. Cell Res.231, 123.
Talanian, R.V., et al. 1997. J. Biol. Chem. 272, 9677.
Thornberry, N.A., et al. 1997. J. Biol. Chem. 272, 17907.
Nicholson, D.W., et al. 1995. Nature376, 37.
Thornberry, N.A., et al. 1992. Nature356, 768.
Datta, R., et al. 1997. J. Biol. Chem. 272, 1965.
Martin, L.M., et al. 1997. J. Biol. Chem. 272, 7421.
Takahashi, A., et al. 1997. Exp. Cell Res.231, 123.
Talanian, R.V., et al. 1997. J. Biol. Chem. 272, 9677.
Thornberry, N.A., et al. 1997. J. Biol. Chem. 272, 17907.
Nicholson, D.W., et al. 1995. Nature376, 37.
Thornberry, N.A., et al. 1992. Nature356, 768.
法律資訊
CALBIOCHEM is a registered trademark of Merck KGaA, Darmstadt, Germany
儲存類別代碼
10 - Combustible liquids
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门