208743
Calpain Inhibitor XI
The Calpain Inhibitor XI, also referenced under CAS 145731-49-3, controls the biological activity of Calpain. This small molecule/inhibitor is primarily used for Protease Inhibitors applications.
别名:
Calpain Inhibitor XI, Z-L-Abu-CONH(CH₂)₃-morpholine
登录查看公司和协议定价
所有图片(1)
About This Item
推荐产品
品質等級
化驗
≥95% (HPLC)
形狀
solid
製造商/商標名
Calbiochem®
儲存條件
OK to freeze
desiccated (hygroscopic)
顏色
white to off-white
溶解度
DMSO: 5 mg/mL
運輸包裝
ambient
儲存溫度
2-8°C
一般說明
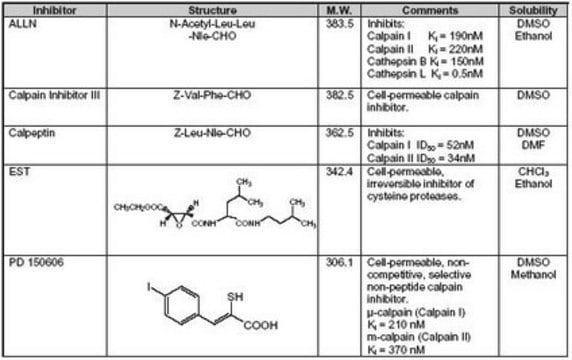
A cell-permeable dipeptidyl α-ketoamide that acts as a potent, highly selective, reversible, and active site inhibitor of calpain-1 and -2 (Ki = 140 nM and 41 nM, respectively). Weakly inhibits cathepsin B (Ki = 6.9 µM). Reported to have a neuroprotective role in the central nervous system following focal ischemia. Also protects against virus-induced apoptotic myocardial injury in mice.
A cell-permeable dipeptidyl a-ketoamide that acts as a potent, highly selective, reversible, active site inhibitor of calpain-1 (Ki = 140 nM) and calpain-2 (Ki = 41 nM). Weakly inhibits cathepsin B (Ki = 6.9 µM). Shown to inhibit calpain-m-mediated degradation of neurofilament protein (NFP) (IC50 = 600 nM). Also shown to exhibit neuroprotective effects in the central nervous system following focal ischemia. Reported to protect against reovirus-induced myocarditis in mice.
生化/生理作用
Cell permeable: yes
Primary Target
calpain 1, calpain 2
calpain 1, calpain 2
Product does not compete with ATP.
Reversible: yes
Target Ki: 140 nM and 41 nM, against calpain-1 and -2, respectively
包裝
Packaged under inert gas
警告
Toxicity: Standard Handling (A)
序列
Z-Leu-Abu-CONH(CH₂)₃-morpholine (Abu = α-aminobutyric acid)
外觀
Supplied as a trifluoroacetate salt.
重構
Following reconstitution aliquot and freeze (-20°C). Stock solutions are stable for up to 3 months at -20°C.
其他說明
Blomgren, K., et al. 2001. J. Biol. Chem.276, 10191.
DeBiasi, R.L., et al. 2001. J. Virol.75, 351.
Saatman, K.E., et al. 2000. J. Cereb. Blood Flow Metab.20, 66.
Stelmasiak, Z., et al. 2000. Med. Sci. Monit.6, 426.
Blomgren, K., et al. 1999. J. Biol. Chem.274, 14046.
James, T., et al. 1998. J. Neurosci. Res.51, 218.
Li, Z., et al. 1996. J. Med. Chem.39, 4089.
Saatman, K.E, et al. 1996. Proc. Natl. Acad. Sci. USA93, 3428.
Bartus, R.T., et al. 1995. Neurol. Res.17, 249.
Bartus, R.T., et al. 1994. Stroke25, 2265.
DeBiasi, R.L., et al. 2001. J. Virol.75, 351.
Saatman, K.E., et al. 2000. J. Cereb. Blood Flow Metab.20, 66.
Stelmasiak, Z., et al. 2000. Med. Sci. Monit.6, 426.
Blomgren, K., et al. 1999. J. Biol. Chem.274, 14046.
James, T., et al. 1998. J. Neurosci. Res.51, 218.
Li, Z., et al. 1996. J. Med. Chem.39, 4089.
Saatman, K.E, et al. 1996. Proc. Natl. Acad. Sci. USA93, 3428.
Bartus, R.T., et al. 1995. Neurol. Res.17, 249.
Bartus, R.T., et al. 1994. Stroke25, 2265.
法律資訊
CALBIOCHEM is a registered trademark of Merck KGaA, Darmstadt, Germany
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 1
閃點(°F)
Not applicable
閃點(°C)
Not applicable
相关内容
Select different protease inhibitor types based on your needs to prevent protein degradation during isolation and characterization and safeguard proteins in sample prep.
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门