推荐产品
生物来源
synthetic
质量水平
方案
96%
折射率
n20/D 1.465 (lit.)
沸点
88-91.5 °C (lit.)
密度
1.065 g/mL at 25 °C (lit.)
应用
flavors and fragrances
文件
see Safety & Documentation for available documents
食品过敏原
no known allergens
性状检查
meaty; roasted
SMILES字符串
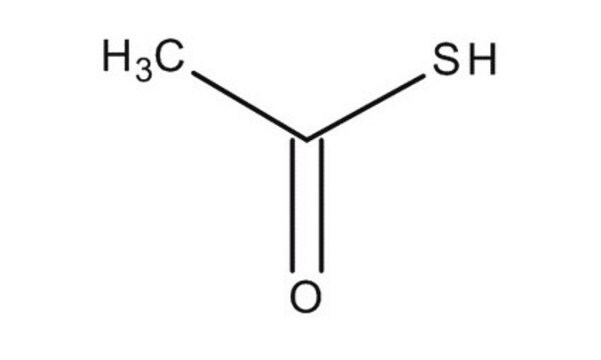
CC(S)=O
InChI
1S/C2H4OS/c1-2(3)4/h1H3,(H,3,4)
InChI key
DUYAAUVXQSMXQP-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
免责声明
For R&D or non-EU Food use. Not for retail sale.
警示用语:
Danger
危险分类
Acute Tox. 3 Oral - Acute Tox. 4 Inhalation - Eye Dam. 1 - Flam. Liq. 2 - Skin Sens. 1
储存分类代码
3 - Flammable liquids
WGK
WGK 3
闪点(°F)
64.4 °F - closed cup
闪点(°C)
18 °C - closed cup
个人防护装备
Faceshields, Gloves, Goggles, type ABEK (EN14387) respirator filter
Julián Valero et al.
Organic & biomolecular chemistry, 10(28), 5417-5430 (2012-06-14)
Polycationic oligo(chiral bicyclic guanidines) constitute useful non-peptidic penetrating agents for cell uptake and protein surface recognition. We report herein improved and selective procedures for the preparation of oligoguanidinium scaffolds linked through thioether bonds, with similar or different groups and functions
Francesco Cellesi et al.
Biomaterials, 25(21), 5115-5124 (2004-04-28)
We have previously described a gelation process based on the occurrence of both physical and a chemical mechanisms ('tandem process'), in which a telechelic linear poly(propylene glycol)-bl-poly(ethylene glycol)-bl-poly(propylene glycol) is first thermally gelled and subsequently covalently cross-linked by the reaction
Rubén Mas-Ballesté et al.
Dalton transactions (Cambridge, England : 2003), 39(6), 1511-1518 (2010-01-28)
While M-percarboxylato species are elusive intermediates, their sulfur containing analogues are known in some cases. The feasibility of isolation of M-perthioacetato compounds allowed, in this work, to obtain new insights into the pathways that follow the reactivity of M-E-ER (E
Wen Yang et al.
Organic & biomolecular chemistry, 10(34), 6876-6884 (2012-08-01)
A highly enantio- and diastereoselective sulfa-Michael addition of thioacetic acid to α,β-disubstituted nitroalkenes catalysed by a chiral squaramide organocatalyst has been described. This organocatalytic reaction at an extremely low catalyst loading (0.2 mol%) furnished synthetically useful β-nitro sulfides in excellent
David Crich et al.
Angewandte Chemie (International ed. in English), 48(13), 2355-2358 (2009-02-21)
Highly activated thioesters formed by the rapid reaction of C-terminal thioacids derived from protected amino acids and peptides with the Sanger reagent and other electron-deficient aryl halides in the presence of a free amine immediately form a peptide bond with
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系客户支持