推荐产品
一般說明
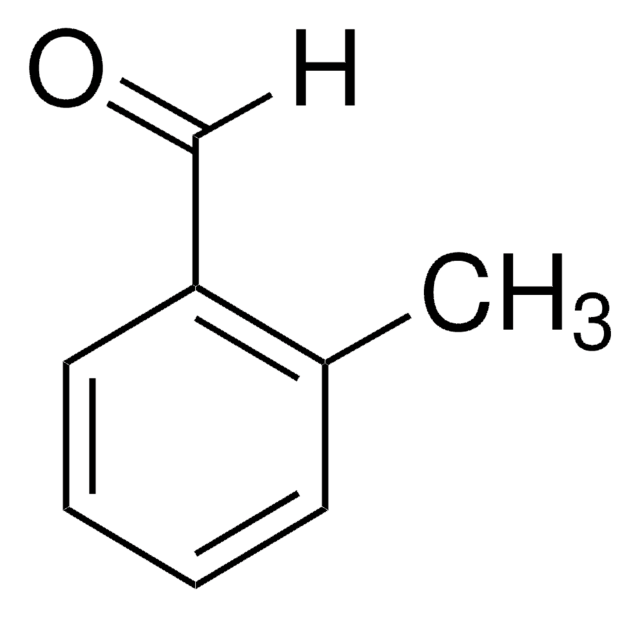
对甲基苯甲醛(4-甲基苯甲醛)是一种芳香醛。它是对二甲苯在紫外光照射氧化过程中通过光致电子转移机制产生的主要氧化产物。它与二铁μ-次乙基复合物发生缩合反应,得到 μ-乙烯基卡拜配合物(产率92%)。
應用
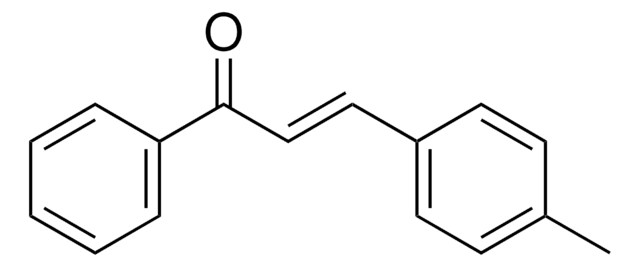
对甲基苯甲醛可用于制备(E)-3-(4-甲基-苯基)-1-(1,3-噻唑-2-基)丙-2-烯-1-酮。它可用于制备(Z)-三取代的烯丙醇。
对甲基苯甲醛可用作恶臭假单胞菌(Pseudomonas putida)菌株培养基中的生长底物。
訊號詞
Warning
危險聲明
危險分類
Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2
儲存類別代碼
10 - Combustible liquids
水污染物質分類(WGK)
WGK 1
閃點(°F)
161.6 °F - closed cup
閃點(°C)
72 °C - closed cup
個人防護裝備
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
其他客户在看
Annamalai Palaniappan et al.
Acta crystallographica. Section E, Structure reports online, 68(Pt 6), o1875-o1875 (2012-06-22)
In the title chalcone, C(13)H(11)NOS, derived from the condensation of p-tolualdehyde and 1-(1,3-thia-zol-2-yl)ethanone, the olefine group has a trans configuration. No classical hydrogen bonding is present in the crystal structure.
Ohkubo et al.
Organic letters, 2(23), 3647-3650 (2000-11-14)
The 100% selective oxygenation of p-xylene to p-tolualdehyde is initiated by photoinduced electron transfer from p-xylene to the singlet excited state of 10-methyl-9-phenylacridinium ion under visible light irradiation, yielding p-tolualdehyde exclusively as the final oxygenated product. The reason for the
Young K Chen et al.
Journal of the American Chemical Society, 126(12), 3702-3703 (2004-03-25)
In this Communication, we outline a new one-pot, multicomponent coupling reaction that allows easy access to (Z)-trisubstituted allylic alcohols. Our strategy is based on E to Z isomerization of the 1-bromo-1-dialkylvinylborane upon reaction with dialkylzinc reagents, and subsequent transmetalation to
M J Worsey et al.
Journal of bacteriology, 124(1), 7-13 (1975-10-01)
Pseudomonas putida (arvilla) mt-2 carries genes for the catabolism of toluene, m-xylene, and p-xylene on a transmissible plasmid, TOL. These compounds are degraded by oxidation of one of the methyl substituents via the corresponding alcohols and aldehydes to benzoate and
Synthesis of cationic diiron μ-vinylcarbyne complexes.
Casey CP, et al.
Polyhedron, 7(10), 881-992 (1988)
实验方案
-Tolualdehyde; Valeraldehyde; Isovaleraldehyde
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门