推荐产品
ligand
thalidomide
品質等級
化驗
≥95%
形狀
powder
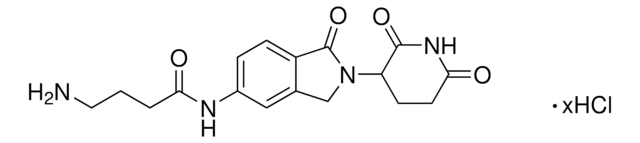
SMILES 字串
O=C1C2=CC=C(N)C=C2C(=O)N1C3C(=O)NC(=O)CC3
InChI
1S/C13H11N3O4/c14-6-1-2-7-8(5-6)13(20)16(12(7)19)9-3-4-10(17)15-11(9)18/h1-2,5,9H,3-4,14H2,(H,15,17,18)
InChI 密鑰
IICWMVJMJVXCLY-UHFFFAOYSA-N
應用
5-Amino-Thalidomide is a derivative of the Cereblon (CRBN)-binding ligand thalidomide. 5-Amino-Thalidomide can be used to bind the CRBN E3 ubiquitin ligase in targeted protein degradation research or in the synthesis
of protein degraders with a C5 exit vector. This was previously listed under AMBH2D6FF88F.
Related Technology Spotlight: Degrader Building Blocks for Targeted Protein Degradation
Browse our growing synthesis and research tools: Protein Degrader Building Blocks
of protein degraders with a C5 exit vector. This was previously listed under AMBH2D6FF88F.
Related Technology Spotlight: Degrader Building Blocks for Targeted Protein Degradation
Browse our growing synthesis and research tools: Protein Degrader Building Blocks
其他說明
Discovery of novel resorcinol diphenyl ether-based PROTAC-like molecules as dual inhibitors and degraders of PD-L1
Efficient Synthesis of Immunomodulatory Drug Analogues Enables Exploration of Structure-Degradation Relationships
Targeting the C481S Ibrutinib-Resistance Mutation in Bruton′s Tyrosine Kinase Using PROTAC-Mediated Degradation
Efficient Synthesis of Immunomodulatory Drug Analogues Enables Exploration of Structure-Degradation Relationships
Targeting the C481S Ibrutinib-Resistance Mutation in Bruton′s Tyrosine Kinase Using PROTAC-Mediated Degradation
法律資訊
PROTAC is a registered trademark of Arvinas Operations, Inc., and is used under license
相關產品
产品编号
说明
价格
訊號詞
Danger
危險聲明
危險分類
Acute Tox. 3 Oral - Acute Tox. 4 Dermal - Repr. 1A
儲存類別代碼
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
Binbin Cheng et al.
European journal of medicinal chemistry, 199, 112377-112377 (2020-05-11)
Novel resorcinol diphenyl ether-based PROTACs (PROteolysis TArgeting Chimeras) were designed and evaluated for their inhibitory activity against the programmed cell death-1/programmed cell death-ligand 1 (PD-1/PD-L1) pathway and their ability to degrade PD-L1 protein. Most of the compounds displayed excellent inhibitory
George M Burslem et al.
ChemMedChem, 13(15), 1508-1512 (2018-06-06)
The immunomodulatory drugs (IMiDs) thalidomide, pomalidomide, and lenalidomide have been approved for the treatment of multiple myeloma for many years. Recently, their use as E3 ligase recruiting elements for small-molecule-induced protein degradation has led to a resurgence in interest in
Alexandru D Buhimschi et al.
Biochemistry, 57(26), 3564-3575 (2018-06-01)
Inhibition of Bruton's tyrosine kinase (BTK) with the irreversible inhibitor ibrutinib has emerged as a transformative treatment option for patients with chronic lymphocytic leukemia (CLL) and other B-cell malignancies, yet >80% of CLL patients develop resistance due to a cysteine
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门