推荐产品
化驗
97%
形狀
sublimed
損耗
0.5 wt. %, 280 °C
半導體屬性
P-type (mobility=0.3 cm2/V·s)
SMILES 字串
c1cc2cc3cc4cc5sccc5cc4cc3cc2s1
InChI
1S/C18H10S2/c1-3-19-17-9-15-8-14-6-12-2-4-20-18(12)10-16(14)7-13(15)5-11(1)17/h1-10H
InChI 密鑰
DAMUWSYTQPWFIY-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
一般說明
應用
商品
Organic materials in optoelectronic devices like LEDs and solar cells are of significant academic and commercial interest.
Intrinsically stretchable active layers for organic field-effect transistors (OFET) are discussed. Polymer structural modification & post-polymerization modifications are 2 methods to achieve this.
Solution-processed organic photovoltaic devices (OPVs) have emerged as a promising clean energy generating technology due to their ease of fabrication, potential to enable low-cost manufacturing via printing or coating techniques, and ability to be incorporated onto light weight, flexible substrates.
Thin, lightweight, and flexible electronic devices meet widespread demand for scalable, portable, and robust technology.
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门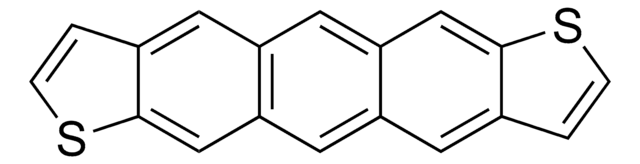
![双萘并 [2,3-b:2′,3′-f] 噻吩并 [3,2-b] 噻吩 sublimed grade, 99%](/deepweb/assets/sigmaaldrich/product/structures/196/451/8a650b8e-abbb-4ef1-9be4-73f223165062/640/8a650b8e-abbb-4ef1-9be4-73f223165062.png)



![噻吩并[3,2-b]噻吩 95%](/deepweb/assets/sigmaaldrich/product/structures/353/609/429fd4bf-e217-4371-80a3-9e5a4d88908b/640/429fd4bf-e217-4371-80a3-9e5a4d88908b.png)



