所有图片(2)
About This Item
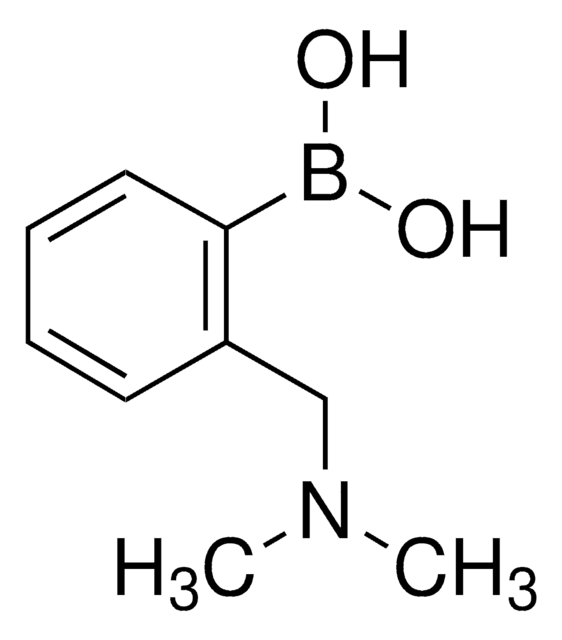
经验公式(希尔记法):
C8H12BNO2
CAS号:
分子量:
165.00
MDL號碼:
分類程式碼代碼:
12352103
PubChem物質ID:
NACRES:
NA.22
推荐产品
化驗
≥95%
mp
178-190 °C
SMILES 字串
CN(C)c1cccc(c1)B(O)O
InChI
1S/C8H12BNO2/c1-10(2)8-5-3-4-7(6-8)9(11)12/h3-6,11-12H,1-2H3
InChI 密鑰
YZQQHZXHCXAJAV-UHFFFAOYSA-N
一般說明
可含不定量的酸酐
應用
Reactant involved in synthesis of different protein effector including:
Reactant involved in synthesis of:
Reactant to undergo regioselective iodination and bromination
- Modulators of survival motor neuron protein
- Glucokinase activators
- Aryl ethers for use as Bacillus anthracis enoyl-ACP reductase inhibitors
Reactant involved in synthesis of:
- Thiourea-functionalized paracyclophanes
- Low-background fluorescent imaging agents for nitric oxide
Reactant to undergo regioselective iodination and bromination
訊號詞
Warning
危險聲明
危險分類
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
dust mask type N95 (US), Eyeshields, Gloves
Gözde Murat Saltan et al.
Spectrochimica acta. Part A, Molecular and biomolecular spectroscopy, 188, 372-381 (2017-08-02)
In an approach to develop efficient organic optoelectronic devices to be used in light-driven systems, a series of three thiophene linked benzimidazole conjugates were synthesized and characterized. The combination of two thiophene rings to a benzimidazole core decorated with different
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门
![[1,1′-双(二苯基膦)二茂铁]二氯化钯(II)](/deepweb/assets/sigmaaldrich/product/structures/130/734/8846aa26-1858-458a-998d-8c306c13bf0f/640/8846aa26-1858-458a-998d-8c306c13bf0f.png)

![[1,1′-双(二苯基膦)二茂铁]二氯化钯(II)二氯甲烷络合物](/deepweb/assets/sigmaaldrich/product/structures/825/986/4317978b-1256-4c82-ab74-6a6a3ef948b1/640/4317978b-1256-4c82-ab74-6a6a3ef948b1.png)