推荐产品
等級
technical grade
光學純度
enantiomeric excess: ≥99.0% (HPLC)
mp
46-55 °C
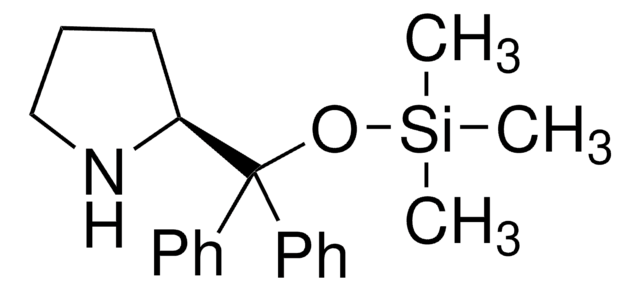
SMILES 字串
C[Si](C)(C)OC([C@H]1CCCN1)(c2cc(cc(c2)C(F)(F)F)C(F)(F)F)c3cc(cc(c3)C(F)(F)F)C(F)(F)F
InChI
1S/C24H23F12NOSi/c1-39(2,3)38-20(19-5-4-6-37-19,13-7-15(21(25,26)27)11-16(8-13)22(28,29)30)14-9-17(23(31,32)33)12-18(10-14)24(34,35)36/h7-12,19,37H,4-6H2,1-3H3/t19-/m1/s1
InChI 密鑰
MOHRGTBNEJKFMB-LJQANCHMSA-N
應用
- Cyclocondensation of enals with methylenepyrrolidines
- Organocatalytic additions of β-ketosulfoxides to conjugated aldehydes
- Organocatalytic aza-Michael reactions
- Stereoselective propargylic alkylation of propargylic esters with aldehydes
- Epoxidation or aziridination of α,β-unsaturated aldehydes and Feist-Benary reactions of 1,3-dicarbonyls
訊號詞
Warning
危險聲明
危險分類
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
>230.0 °F - closed cup
閃點(°C)
> 110 °C - closed cup
個人防護裝備
dust mask type N95 (US), Eyeshields, Gloves
其他客户在看
商品
Professor Karl Anker Jørgensen and his group have developed ethers which serve as excellent chiral organocatalysts in the direct asymmetric α-functionalization of aldehydes.
Professor Geoffrey Coates and co-workers at Cornell University have reported the preparation and use of catalysts composed of an oxophilic Lewis acid and a cobalt tetracarbonyl anion for the ring expansive carbonylation of epoxides to b-lactones and b-lactones to succinic anhydrides.
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门![(S)-α,α-双 [3,5-双(三氟甲基)苯基]-2-吡咯烷甲醇三甲基硅醚 97%](/deepweb/assets/sigmaaldrich/product/structures/396/398/09a397b1-b5f5-420f-98da-adf9017cef56/640/09a397b1-b5f5-420f-98da-adf9017cef56.png)


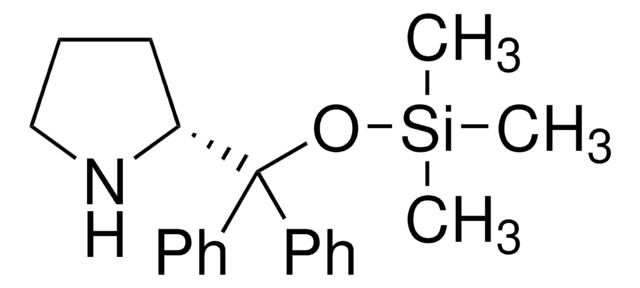
![(S)-α,α-双[3,5-双(三氟甲基)苯基]-2-吡咯烷甲醇 ≥99.0%](/deepweb/assets/sigmaaldrich/product/structures/201/440/11d18670-8609-4657-bb4b-af6c424f8791/640/11d18670-8609-4657-bb4b-af6c424f8791.png)
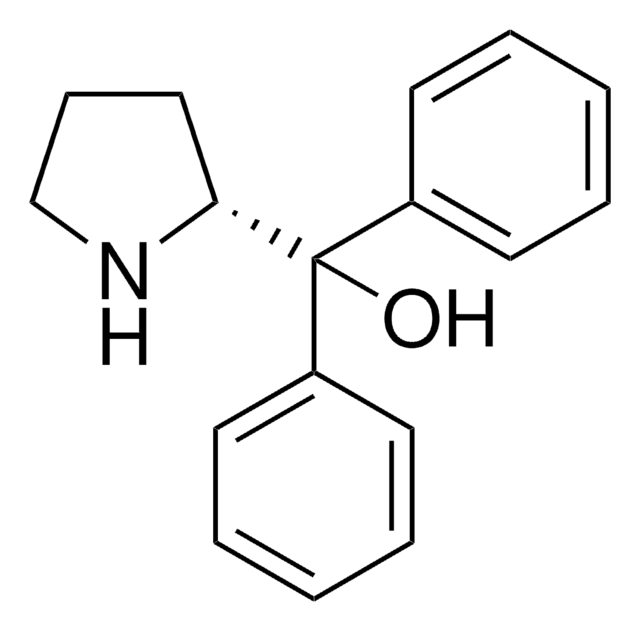

![(S)-2-[[3,5-双(三氟甲基)苯基]硫脲基]-N-苄基-N,3,3-三甲基丁酰胺 97%](/deepweb/assets/sigmaaldrich/product/structures/373/888/118b46f2-6c2e-4a87-8266-c4dbcd5db51f/640/118b46f2-6c2e-4a87-8266-c4dbcd5db51f.png)


