所有图片(1)
About This Item
经验公式(希尔记法):
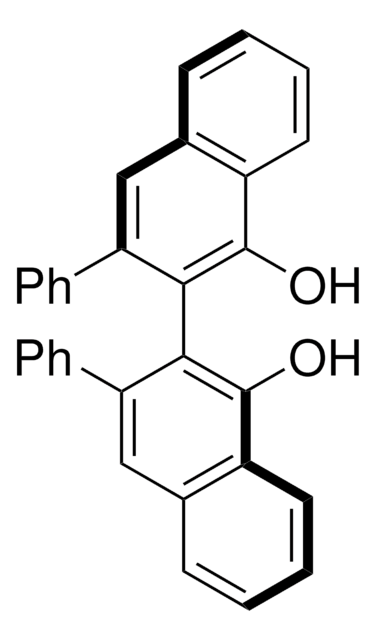
C32H22O2
CAS号:
分子量:
438.52
MDL號碼:
分類程式碼代碼:
12352005
PubChem物質ID:
NACRES:
NA.22
推荐产品
化驗
97%
形狀
solid
光學活性
[α]20/D +310°, c = 1 in chloroform
mp
200-204 °C
SMILES 字串
Oc1c(c(cc2ccccc12)-c3ccccc3)-c4c(O)c5ccccc5cc4-c6ccccc6
InChI
1S/C32H22O2/c33-31-25-17-9-7-15-23(25)19-27(21-11-3-1-4-12-21)29(31)30-28(22-13-5-2-6-14-22)20-24-16-8-10-18-26(24)32(30)34/h1-20,33-34H
InChI 密鑰
NDTDVKKGYBULHF-UHFFFAOYSA-N
應用
已证实 VANOL 是用于催化型不对称 Diels-Alder 反应、亚胺羟醛反应以及氮杂环丙化反应的绝佳配体。
(R)-VANOL is a vaulted biaryl ligand that may be used in the following processes:
- Transformation of 3-substituted cyclobutanones to enantiopure γ-butyrolactones via asymmetric Baeyer-Villiger Reaction in the presence of an aluminum catalyst.
- Asymmetric aziridination of N-(4-(Methylsulfonyl)benzylidene)diphenylmethanamine to form (2S,3S)-ethyl 1-benzhydryl-3-(4-(methylsulfonyl)phenyl) aziridine-2-carboxylate.
- Conjugate addition of terminal alkynes to 2-arylidene-1,3-diketones with high enantioselectivity in the presence of diethyl zinc.
訊號詞
Danger
危險分類
Aquatic Chronic 4 - Eye Dam. 1 - Skin Irrit. 2 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
dust mask type N95 (US), Eyeshields, Gloves
其他客户在看
Blay G, et al.
Chemistry?A European Journal , 18(41), 12966-12969 (2012)
Vaulted biaryls: efficient ligands for the aluminum-catalyzed asymmetric Baeyer-Villiger reaction.
Bolm C, et al.
Synlett, 2004(09), 1619-1621 (2004)
An efficient enantioselective synthesis of florfenicol via asymmetric aziridination.
Wang Z, et al.
Tetrahedron, 67(47), 9199-9203 (2011)
Bao. J. et al.
Journal of the American Chemical Society, 118, 3392-3392 (1996)
Su Yu et al.
Organic letters, 7(3), 367-369 (2005-01-28)
[reaction: see text] In an effort to develop a synthesis of the VAPOL ligand that avoids the use of a chromium carbene complex, a route was examined that involved the annulation of a naphthalene carboxamide via the method of Snieckus.
相关内容
The research areas of interest to the Wulff group include enantioselective catalysis, mechanisms of asymmetric catalysis, macromolecular chemistry, Fischer carbene complexes in organic synthesis and the total synthesis of natural products.
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门