所有图片(1)
About This Item
线性分子式:
BrC6H3(CF3)SO2Cl
CAS号:
分子量:
323.51
MDL號碼:
分類程式碼代碼:
12352100
PubChem物質ID:
NACRES:
NA.22
推荐产品
化驗
97%
折射率
n20/D 1.5270 (lit.)
bp
235-236 °C (lit.)
密度
1.865 g/mL at 25 °C (lit.)
SMILES 字串
FC(F)(F)c1ccc(c(Br)c1)S(Cl)(=O)=O
InChI
1S/C7H3BrClF3O2S/c8-5-3-4(7(10,11)12)1-2-6(5)15(9,13)14/h1-3H
InChI 密鑰
WWEGTYZLWBOTQW-UHFFFAOYSA-N
應用
2-Bromo-4-(trifluoromethyl)benzenesulfonyl chloride may be used to synthesize butyl (E)-3-[2-bromo-4-(trifluoromethyl)phenyl]acrylate and 1-benzyl-2-(2-bromo-4-(trifluoromethyl)phenyl)indole.
訊號詞
Danger
危險聲明
危險分類
Flam. Liq. 3 - Skin Corr. 1B
儲存類別代碼
3 - Flammable liquids
水污染物質分類(WGK)
WGK 3
閃點(°F)
95.0 °F - closed cup
閃點(°C)
35 °C - closed cup
個人防護裝備
Faceshields, Gloves, Goggles, type ABEK (EN14387) respirator filter
Palladium-Catalysed Desulfitative Heck Reaction Tolerant to Aryl Carbon-Halogen Bonds for Access to (Poly) halo-Substituted Stilbene or Cinnamate Derivatives.
Skhiri A, et al.
Synthesis (2016)
Anoir Hfaiedh et al.
Organic & biomolecular chemistry, 14(21), 4947-4956 (2016-05-14)
The direct arylation of N-protected 3-haloindole derivatives with benzenesulfonyl chlorides as coupling partners using 5 mol% of bis(acetonitrile)dichloropalladium(ii) catalyst and lithium carbonate as a base in 1,4-dioxane was investigated. We demonstrated that both iodo and chloro substituents at the indolyl
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门


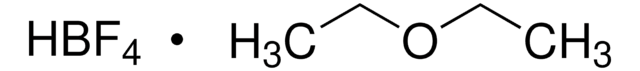
![(-)-顺-6,6-二甲基双环[3.1.1]-2-氨甲基 98%](/deepweb/assets/sigmaaldrich/product/structures/249/650/f951a825-0713-438b-b239-0f059326bff3/640/f951a825-0713-438b-b239-0f059326bff3.png)






