所有图片(1)
About This Item
经验公式(希尔记法):
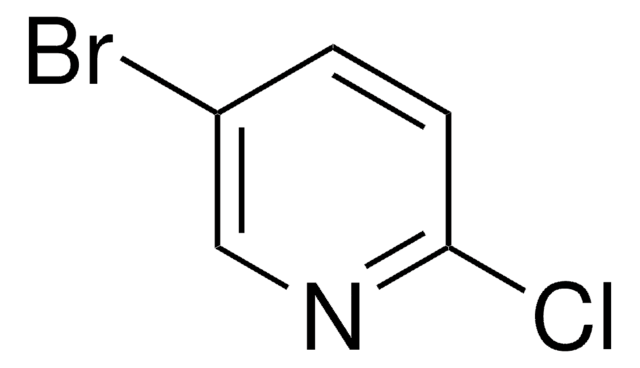
C5H3BrClN
CAS号:
分子量:
192.44
MDL號碼:
分類程式碼代碼:
12352100
PubChem物質ID:
NACRES:
NA.22
推荐产品
化驗
98%
形狀
solid
mp
54-57 °C (lit.)
SMILES 字串
Clc1ncccc1Br
InChI
1S/C5H3BrClN/c6-4-2-1-3-8-5(4)7/h1-3H
InChI 密鑰
HDYNIWBNWMFBDO-UHFFFAOYSA-N
一般說明
3-Bromo-2-chloropyridine can be synthesized from 3-amino-2-chloropyridine or 2-chloro-3-pyridinamine.
應用
3-Bromo-2-chloropyridine may be used to synthesize:
- acetylenic dipyridone
- 3-ethynyl-2-(phenylmethoxy)-pyridine
- nemertelline
- ortho-chlorodiheteroarylamine4 or 2-chloro-N-(2,3,7-trimethylbenzo[b]thien-6-yl)pyridin-3-amine
訊號詞
Warning
危險聲明
危險分類
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
個人防護裝備
dust mask type N95 (US), Eyeshields, Gloves
Maria-João R P Queiroz et al.
Bioorganic & medicinal chemistry, 14(20), 6827-6831 (2006-07-18)
ortho-Chlorodiarylamines in the 2,3,7-trimethylbenzo[b]thiophene series were prepared in high yields (70-85%) by C-N palladium-catalyzed cross-coupling using P(t-Bu)(3) as ligand and NaOt-Bu as base. A palladium-assisted C-C intramolecular cyclization of the coupling products gave thienocarbazoles and the dechlorinated diarylamines. Studies of
Use of hydrogen bonds to control molecular aggregation. behavior of dipyridones and pyridone-pyrimidones designed to form cyclic triplexes.
Boucher E, et al.
The Journal of Organic Chemistry, 60(5), 1408-1412 (1995)
Synthesis of the first thieno-d-carboline: Fluorescence studies in solution and in lipid vesicles.
Queiroz MJRP, et al.
Journal of Photochemistry and Photobiology A: Chemistry, 181(2), 290-296 (2006)
Synthesis of novel halopyridinylboronic acids and esters. Part 2: 2, 4, or 5-Halopyridin-3-yl-boronic acids and esters.
Bouillon A, et al.
Tetrahedron, 58(17), 3323-3328 (2002)
Alexandre Bouillon et al.
The Journal of organic chemistry, 68(26), 10178-10180 (2003-12-20)
Regioselective and univocal Suzuki cross-coupling reactions performed on halopyridinyl boronic acids provide a flexible and versatile route to a multigram scale synthesis of 2,2'-dichloro-3,4'-bipyridine 14, which allows couplings with excess pyridin-3-yl boronic acid to give a new and efficient two-step
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门



![(Ir[dF(CF3)ppy]2(dtbpy))PF6](/deepweb/assets/sigmaaldrich/product/structures/982/913/02dd8ddd-6deb-40a0-ab9b-07b18f1abb09/640/02dd8ddd-6deb-40a0-ab9b-07b18f1abb09.png)