推荐产品
化驗
97%
mp
57-60 °C (lit.)
儲存溫度
2-8°C
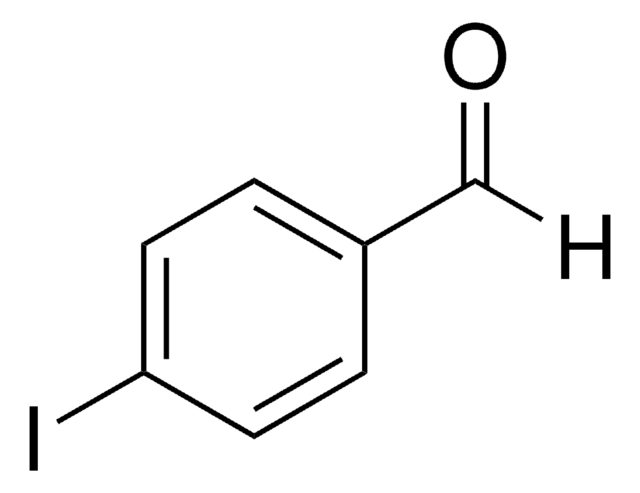
SMILES 字串
Ic1cccc(C=O)c1
InChI
1S/C7H5IO/c8-7-3-1-2-6(4-7)5-9/h1-5H
InChI 密鑰
RZODAQZAFOBFLS-UHFFFAOYSA-N
一般說明
3-Iodobenzaldehyde can be prepared by the iodination of benzaldehyde using 1,3-diiodo-5,5-dimethylhydantoin in sulfuric acid.
應用
3-Iodobenzaldehyde may be used as a starting material in the preparation of:
- 3-iodocinnamic acid
- 3-(3-hydroxy-3-methylbut-1-yn-1-yl)benzaldehyde
- 1,3-dihydroxy-2-(3-iodophenyl)-4,4,5,5-tetramethylimidazolidine
訊號詞
Warning
危險聲明
危險分類
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
dust mask type N95 (US), Eyeshields, Gloves
其他客户在看
1, 3-Diiodo-5,5-dimethylhydantoin-An efficient reagent for iodination of aromatic compounds.
Chaikovskii VK, et al.
Russ. J. Org. Chem., 43(9), 1291-1296 (2007)
Suzuki Reactions with Stable Organic Radicals-Synthesis of Biphenyls Substituted with Nitronyl-Nitroxide Radicals.
Stroh C, et al.
European Journal of Organic Chemistry, 2005(17), 3697-3703 (2005)
Cross-coupling of aryl iodides with paramagnetic terminal acetylenes derived from 4, 4, 5, 5-tetramethyl-2-imidazoline-1-oxyl 3-oxide.
Klyatskaya SV, et al.
Russian Chemical Bulletin, 51(1), 128-134 (2002)
Synthesis and evaluation of potent and selective human V1a receptor antagonists as potential ligands for PET or SPECT imaging.
Fabio K, et al.
Bioorganic & Medicinal Chemistry, 20(3), 1337-1345 (2012)
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门