所有图片(1)
About This Item
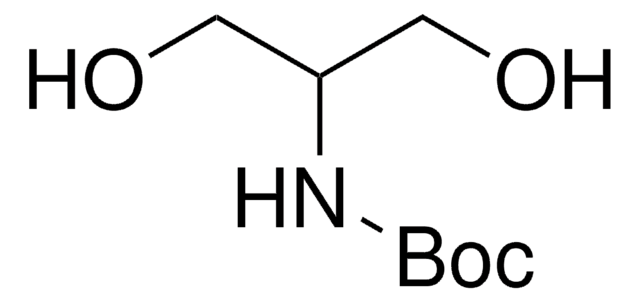
线性分子式:
HOCH2CH2CH[NHCO2C(CH3)3]CH2OH
CAS号:
分子量:
205.25
MDL编号:
UNSPSC代码:
12352108
PubChem化学物质编号:
NACRES:
NA.22
推荐产品
质量水平
方案
97%
旋光性
[α]20/D −8°, c = 1 in chloroform
mp
65-69 °C (lit.)
官能团
amine
hydroxyl
SMILES字符串
CC(C)(C)OC(=O)N[C@H](CO)CCO
InChI
1S/C9H19NO4/c1-9(2,3)14-8(13)10-7(6-12)4-5-11/h7,11-12H,4-6H2,1-3H3,(H,10,13)/t7-/m0/s1
InChI key
KLRRFBSWOIUAHZ-ZETCQYMHSA-N
应用
(S)-(−)-2-(Boc-amino)-1,4-butanediol can be used as a reactant to synthesize:
- Thiourea-based organocatalysts for asymmetric Michael addition reactions of nitroalkenes to α-nitrocyclohexanone.
- Bis-copper (II) complex based catalysts for enantioselective Michael reactions.
储存分类代码
11 - Combustible Solids
WGK
WGK 3
闪点(°F)
Not applicable
闪点(°C)
Not applicable
个人防护装备
Eyeshields, Gloves, type N95 (US)
Asymmetric Michael additions of α-nitrocyclohexanone to aryl nitroalkenes catalyzed by natural amino acid-derived bifunctional thioureas
Jo?rres M, et al.
Organic Letters, 14(17), 4518-4521 (2012)
Copper (II) in organic synthesis. XI. Evaluation of the ligand architecture on the efficiency of a copper (II) catalyst for enantioselective Michael reactions
Desimoni G, et al.
Tetrahedron, 51(14), 4131-4144 (1995)
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系客户支持