推荐产品
化驗
98%
mp
73-76 °C (lit.)
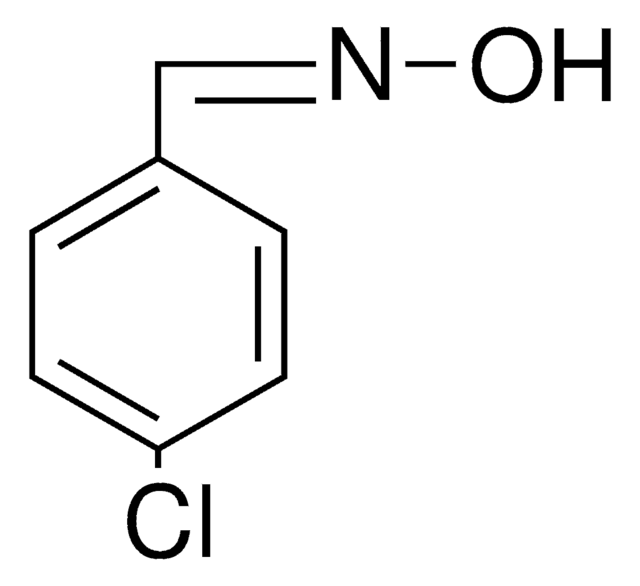
SMILES 字串
O\N=C\c1ccccc1Cl
InChI
1S/C7H6ClNO/c8-7-4-2-1-3-6(7)5-9-10/h1-5,10H/b9-5+
InChI 密鑰
FZIVKDWRLLMSEJ-WEVVVXLNSA-N
一般說明
2-Chlorobenzaldehyde oxime is also known as o-chlorobenzaldehyde oxime. It can be synthesized by reacting 2-chlorobenzaldehyde and hydroxylamine hydrochloride.
應用
2-Chlorobenzaldehyde oxime may be used in the preparation of:
- 2-chlorobenzaldehyde under different reaction conditions
- methyl 3-(2-chlorophenyl)-5-[1-(4-methoxybenzyloxy)-ethyl]isoxazole-4-carboxylate
- dimethyl 3-(2-chlorophenyl)isoxazole-4,5-dicarboxylate
- [3-(2-chlorophenyl)isoxazol-5-yl]methanol
訊號詞
Warning
危險聲明
危險分類
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
dust mask type N95 (US), Eyeshields, Gloves
A mild and selective method for the conversion of oximes into ketones and aldehydes by the use of N-bromophthalimide.
Khazaei A, et al.
J. Chem. Res. (M), 2004(10), 695-696 (2004)
Solid-phase synthesis of 5-isoxazol-4-yl-[1,2,4] oxadiazoles.
Quan C and Kurth M.
The Journal of Organic Chemistry, 69(5), 1470-1474 (2004)
Hypervalent iodine mediated synthesis of di-and tri-substituted isoxazoles via [3+2] cycloaddition of nitrile oxides.
Singhal A, et al.
Tetrahedron, 57(7), 719-722 (2016)
Microwave-assisted chemoselective cleavage of oximes to their corresponding carbonyl compounds using 1, 3-dichloro-5, 5-dimethyl-hydantoin (DCDMH) as a new Deoximating reagent.
Khazaei A and Manesh AA.
Synthesis, 2005(12), 1929-1931 (2005)
Amberlyst 15 supported nitrosonium ion as an efficient reagent for regeneration of carbonyl compounds from oximes, hydrazones and semicarbazones.
Lakouraj MM, et al.
Reactive functional Polymers, 66(9), 910-915 (2006)
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门