所有图片(1)
About This Item
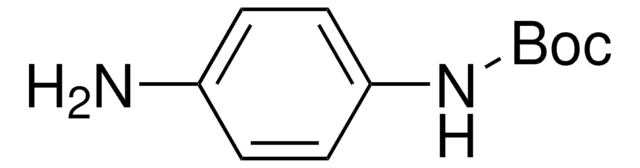
经验公式(希尔记法):
C11H16N2O2
CAS号:
分子量:
208.26
MDL號碼:
分類程式碼代碼:
12352100
PubChem物質ID:
NACRES:
NA.22
推荐产品
化驗
≥98.0% (HPLC)
反應適用性
reagent type: cross-linking reagent
官能基
Boc
amine
SMILES 字串
NC1=CC(NC(OC(C)(C)C)=O)=CC=C1
InChI
1S/C11H16N2O2/c1-11(2,3)15-10(14)13-9-6-4-5-8(12)7-9/h4-7H,12H2,1-3H3,(H,13,14)
InChI 密鑰
IEUIEMIRUXSXCL-UHFFFAOYSA-N
應用
N-Boc-m-phenylenediamine (tert-Butyl-3-aminophenylcarbamate) may be used in the preparation of:
- 5,5′-(propane-2,2-diyl)bis(N-(3-aminophenyl)-4-methyl-3-phenyl-1H-pyrrole-2-carboxamide)
- ethyl 4-[{3-[(tert-butoxycarbonyl)amino]phenyl}amino]-2-chloropyrimidine-5-carboxylate
- ethyl 4-(3-(tert-butoxycarbonyl)phenylamino)-2-(methylthio)pyrimidine-5-carboxylate
訊號詞
Warning
危險聲明
危險分類
Acute Tox. 4 Oral
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
dust mask type N95 (US), Eyeshields, Gloves
Design, synthesis, and biological evaluation of novel conformationally constrained inhibitors targeting epidermal growth factor receptor threonine790? methionine790 mutant.
Chang S, et al.
Journal of Medicinal Chemistry, 55(6), 2711-2723 (2012)
Pyrimido [4,5-d] pyrimidin-4(1H)-one Derivatives as Selective Inhibitors of EGFR Threonine790 to Methionine790 (T790M) Mutants.
Xu T, et al.
Angewandte Chemie (Weinheim an der Bergstrasse, Germany), 125(32), 8545-8548 (2013)
Grigory V Kolesnikov et al.
Organic & biomolecular chemistry, 9(21), 7358-7364 (2011-09-07)
The design and synthesis of a neutral macrocyclic host that is capable of perrhenate and pertechnetate recognition is described. The anion affinities and underlying coordination modes were estimated by several experimental and theoretical methods including a new technique--reverse (99)Tc NMR
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门




![4-[(N-Boc)氨甲基]苯胺 97%](/deepweb/assets/sigmaaldrich/product/structures/341/155/530c425c-7e6e-435e-a28a-9d40b05b938a/640/530c425c-7e6e-435e-a28a-9d40b05b938a.png)

