所有图片(1)
About This Item
经验公式(希尔记法):
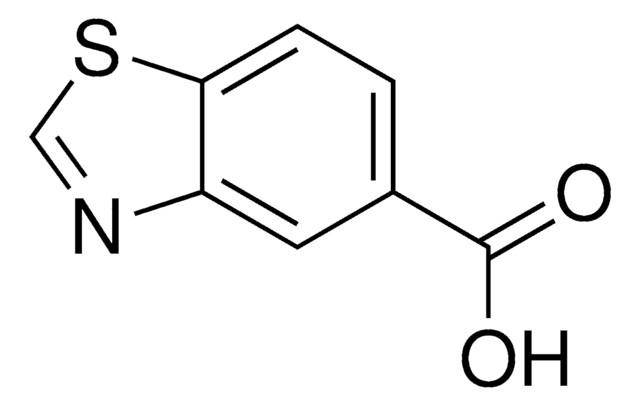
C8H5NO2S
CAS号:
分子量:
179.20
MDL號碼:
分類程式碼代碼:
12352100
PubChem物質ID:
NACRES:
NA.22
推荐产品
化驗
96%
mp
245-251 °C (lit.)
SMILES 字串
OC(=O)c1ccc2ncsc2c1
InChI
1S/C8H5NO2S/c10-8(11)5-1-2-6-7(3-5)12-4-9-6/h1-4H,(H,10,11)
InChI 密鑰
DMPZJACLHDWUFS-UHFFFAOYSA-N
一般說明
苯并噻唑-6-羧酸 (BTCA) 是苯并噻唑衍生物。
應用
苯并噻唑-6-羧酸可用于合成N-(吡啶-4-基)苯并[d]噻唑-6-羧酰胺,表现出了抑制尿路致病性大肠杆菌中K1荚膜形成的潜力。在通过液相色谱-电喷雾电离串联质谱法定量苯并噻唑时,也可用其作内标。
訊號詞
Warning
危險聲明
危險分類
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
dust mask type N95 (US), Eyeshields, Gloves
Yoshihiro Sawada et al.
Pest management science, 59(1), 25-35 (2003-02-01)
The N'-benzoyl group of N-tert-butyl-N'-benzoyl-3,5-dimethylbenzohydrazide (1) was converted to a series of benzoheterocyclecarbonyl groups in order to investigate the potential usefulness of superimposing a hydrazine insecticide on 20-hydroxyecdysone. A series of analogues with benzodioxole, benzodioxane, benzodioxapine, indole, benzoxazole, benzoxazine or
James W. Noah et al.
Probe Reports from the NIH Molecular Libraries Program, 2012 Dec 17 (Updated 2013 Apr 5) (2013-07-09)
Uropathogenic
Stefan Weiss et al.
Analytical chemistry, 77(22), 7415-7420 (2005-11-16)
The first method for the determination of commonly used corrosion inhibitors in environmental water samples by liquid chromatography-electrospray ionization-tandem mass spectrometry is presented. Benzotriazole (BTri) and the two isomers of tolyltriazole (5- and 4-TTri) are separated in an isocratic run.
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门




![5-Bromo-1H-pyrrolo[2,3-b]pyridine-3-carboxylic acid AldrichCPR](/deepweb/assets/sigmaaldrich/product/structures/408/367/d023f137-f9d5-4e3a-bc3a-89b109181588/640/d023f137-f9d5-4e3a-bc3a-89b109181588.png)