所有图片(1)
About This Item
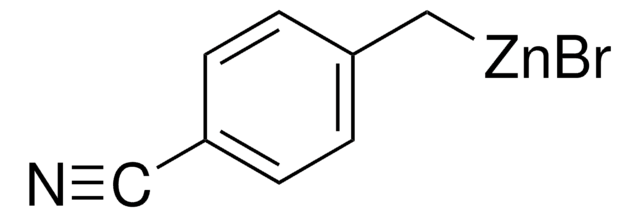
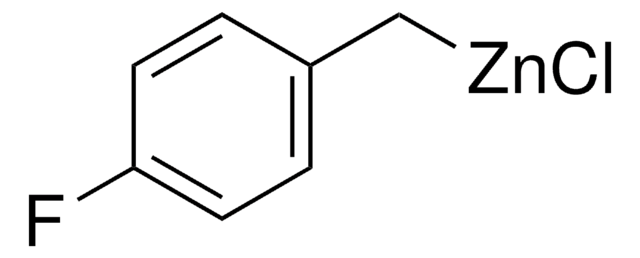
线性分子式:
NCC6H4CH2ZnBr
CAS号:
分子量:
261.43
MDL编号:
UNSPSC代码:
12352103
PubChem化学物质编号:
NACRES:
NA.22
推荐产品
质量水平
浓度
0.5 M in THF
密度
0.993 g/mL at 25 °C
官能团
nitrile
储存温度
2-8°C
SMILES字符串
Br[Zn]Cc1ccccc1C#N
InChI
1S/C8H6N.BrH.Zn/c1-7-4-2-3-5-8(7)6-9;;/h2-5H,1H2;1H;/q;;+1/p-1
InChI key
LQRNYQAXNZETFP-UHFFFAOYSA-M
应用
2-Cyanobenzylzinc bromide can be used as a reactant:
- In the metal-catalyzed Negishi cross-coupling reactions to prepare aryl or heteroaryl derivatives via carbon-carbon bond formation.
- To synthesize 4-(2-Cyanobenzyl)-3′-(trifluoromethyl)biphenyl by reacting with aryl nonaflate in the presence of Pd(dba)2 as a catalyst.
- To prepare 2-[(2-cyanophenyl)methyl] benzamide by treating with 2-iodobenzamide using a nickel catalyst.
法律信息
Rieke® Metals, Inc. 产品 ®Rieke Metals, Inc. 的注册商标
警示用语:
Danger
危险分类
Carc. 2 - Eye Irrit. 2 - Flam. Liq. 2 - STOT SE 3
靶器官
Central nervous system, Respiratory system
补充剂危害
储存分类代码
3 - Flammable liquids
WGK
WGK 3
闪点(°F)
1.4 °F - closed cup
闪点(°C)
-17 °C - closed cup
Improved nickel-catalyzed cross-coupling reaction conditions between ortho-substituted aryl iodides/nonaflates and alkylzinc iodides in solution and in the solid-phase
Jensen AE, et al.
Tetrahedron, 56(25), 4197-4201 (2000)
Visible-Light-Induced Nickel-Catalyzed Negishi Cross-Couplings by Exogenous-Photosensitizer-Free Photocatalysis
Abdiaj I, et al.
Angewandte Chemie (International Edition in English), 57(28), 8473-8477 (2018)
Palladium-catalyzed cross-coupling reactions with aryl nonaflates: a practical alternative to aryl triflates
Rottlander M and Knochel P
The Journal of Organic Chemistry, 63(1), 203-208 (1998)
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门