所有图片(1)
About This Item
经验公式(希尔记法):
C6H6BrNO
CAS号:
分子量:
188.02
MDL號碼:
分類程式碼代碼:
12352100
PubChem物質ID:
NACRES:
NA.22
化驗:
95%
推荐产品
化驗
95%
折射率
n20/D 1.555 (lit.)
bp
80 °C/12 mmHg (lit.)
密度
1.453 g/mL at 25 °C (lit.)
官能基
bromo
SMILES 字串
COc1ccc(Br)cn1
InChI
1S/C6H6BrNO/c1-9-6-3-2-5(7)4-8-6/h2-4H,1H3
InChI 密鑰
XADICJHFELMBGX-UHFFFAOYSA-N
應用
αvβ3 拮抗剂的 β-丙氨酸部分的结构单元也是合成强效选择性促生长素抑制素 sst3 受体拮抗剂的结构单元。
訊號詞
Warning
危險聲明
危險分類
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
10 - Combustible liquids
水污染物質分類(WGK)
WGK 3
閃點(°F)
204.8 °F - closed cup
閃點(°C)
96 °C - closed cup
個人防護裝備
Eyeshields, Gloves, type ABEK (EN14387) respirator filter
历史批次信息供参考:
分析证书(COA)
Lot/Batch Number
其他客户在看
Synthesis and biological evaluation of 1-(benzenesulfonamido)-2-[5-(N-hydroxypyridin-2 (1H)-one)] acetylene regioisomers: A novel class of 5-lipoxygenase inhibitors.
Chowdhury MA, et al.
Bioorganic & Medicinal Chemistry Letters, 18(14), 4195-4198 (2008)
Jiabing Wang et al.
Bioorganic & medicinal chemistry letters, 14(4), 1049-1052 (2004-03-12)
A series of 3-substituted tetrahydro-[1,8]naphthyridine containing alpha(v)beta(3) antagonists was prepared. A comparison of their in vitro IC(50) values to the electron properties of the 3-substituents revealed a good linear Hammett correlation (rho=-1.96, R(2)=0.959). Electron-withdrawing groups at the 3-position of the
Functionalized pyridylboronic acids and their Suzuki cross-coupling reactions to yield novel heteroarylpyridines
Parry PR, et al.
The Journal of Organic Chemistry, 67(21), 7541-7543 (2002)
Negishi Cross-Coupling Reactions Catalyzed by an Aminophosphine-Based Nickel System: A Reliable and General Applicable Reaction Protocol for the High-Yielding Synthesis of Biaryls.
Gerber R and Frech CM.
Chemistry (Weinheim An Der Bergstrasse, Germany), 17(42), 11893-11904 (2011)
Tetrahedron Asymmetry, 14, 3469-3469 (2003)
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门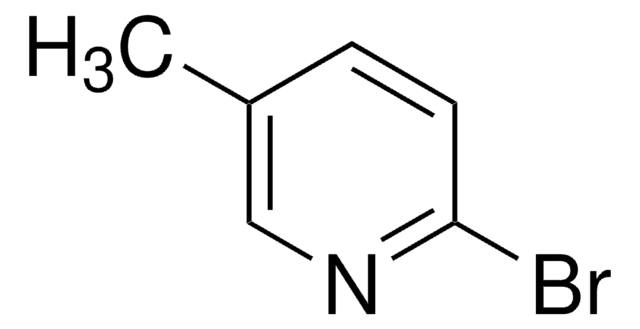








![[Pd2(dba)3] x dba Umicore](/deepweb/assets/sigmaaldrich/product/structures/150/531/11e74f1a-c256-4d30-b43d-8c299f1034b1/640/11e74f1a-c256-4d30-b43d-8c299f1034b1.png)