所有图片(1)
About This Item
经验公式(希尔记法):
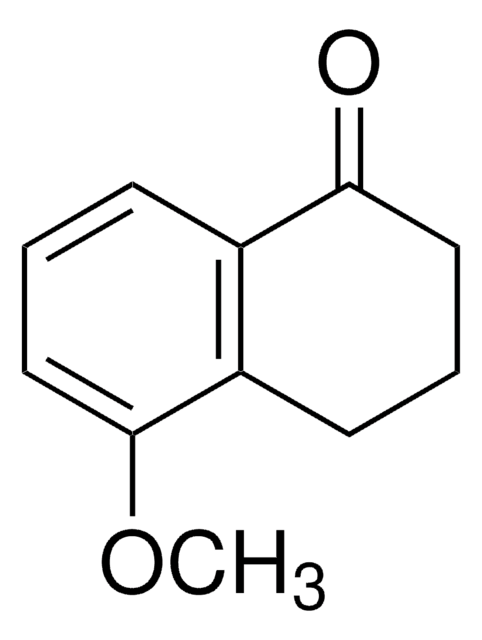
C10H10O2
CAS号:
分子量:
162.19
MDL號碼:
分類程式碼代碼:
12352100
PubChem物質ID:
NACRES:
NA.22
推荐产品
化驗
98%
mp
154-157 °C (lit.)
官能基
ketone
SMILES 字串
Oc1ccc2C(=O)CCCc2c1
InChI
1S/C10H10O2/c11-8-4-5-9-7(6-8)2-1-3-10(9)12/h4-6,11H,1-3H2
InChI 密鑰
FNSQPQKPPGALFA-UHFFFAOYSA-N
一般說明
6-Hydroxy-3,4-dihydro-1(2H)-naphthalenone (6-Hydroxy-1-tetralone) can be prepared by the demethylation of 6-methoxy-3,4-dihydro-1(2H)-naphthalenone. It participates in the synthesis of 2-alkylamino-6-hydroxy-5-hydroxymethyl-1,2,3,4-tetrahydro-1-naphthalenols. Its pKa value has been reported to be 7.74.
應用
6-Hydroxy-3,4-dihydro-1(2H)-naphthalenone (6-Hydroxy-1-tetralone) may be used in the synthesis of:
- 5-chloromethyl-6-hydroxy-3,4-dihydro-1(2H)-naphthalenone
- 6-(4-(3-(piperidin-1-yl)propoxy)benzyloxy)-1-tetralone
- 6-(3-(piperidin-1-yl)propoxy)-1-tetralone
訊號詞
Warning
危險聲明
危險分類
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
個人防護裝備
dust mask type N95 (US), Eyeshields, Gloves
Syntheses and beta-adrenoceptor activities of 2-alkylamino-6-hydroxy-5-hydroxymethyl-1,2,3,4-tetrahydro-1-naphthalenols.
H Sugihara et al.
Chemical & pharmaceutical bulletin, 25(11), 2988-3002 (1977-11-01)
Miriam Tomasch et al.
Frontiers in systems neuroscience, 6, 14-14 (2012-04-04)
Novel fluorescent chalcone-based ligands at human histamine H(3) receptors (hH(3)R) have been designed, synthesized, and characterized. Compounds described are non-imidazole analogs of ciproxifan with a tetralone motif. Tetralones as chemical precursors and related fluorescent chalcones exhibit affinities at hH(3)R in
Ewelina van Wenum et al.
The Journal of organic chemistry, 78(18), 9102-9112 (2013-09-07)
Silybin (SIL) and 2,3-dehydrosilybin (DHS) are constituents of milk thistle extract (silymarin) applied in the treatment of cirrhosis, hepatitis, and alcohol-induced liver disease. The molecular mechanism of their action is usually connected with antioxidant action. However, despite experimental and theoretical
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门