推荐产品
方案
97%
旋光性
[α]20/D −30°, c = 1 in chloroform
反应适用性
reaction type: solution phase peptide synthesis
mp
90-92 °C (lit.)
应用
peptide synthesis
SMILES字符串
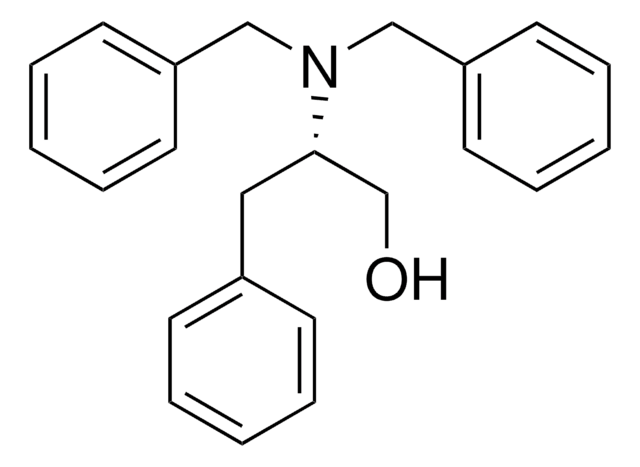
OC[C@H](Cc1ccccc1)NC(=O)OCc2ccccc2
InChI
1S/C17H19NO3/c19-12-16(11-14-7-3-1-4-8-14)18-17(20)21-13-15-9-5-2-6-10-15/h1-10,16,19H,11-13H2,(H,18,20)/t16-/m0/s1
InChI key
WPOFMMJJCPZPAO-INIZCTEOSA-N
应用
用于合成具生物化学活性的化合物。合成 HIV 蛋白酶抑制剂的结构单元。
储存分类代码
11 - Combustible Solids
WGK
WGK 3
闪点(°F)
Not applicable
闪点(°C)
Not applicable
个人防护装备
Eyeshields, Gloves, type N95 (US)
Pierre L. Beaulieu et al.
The Journal of organic chemistry, 61(11), 3635-3645 (1996-05-31)
Enantiomerically pure N,N-dibenzyl-alpha-amino aldehydes reacted with (chloromethyl)lithium, generated in situ from bromochloromethane and lithium metal, to give predominantly erythro aminoalkyl epoxides. Treatment of the crude epoxides with aqueous hydrochloric acid gave crystalline (2S,3S)-N,N-dibenzylamino chlorohydrin hydrochlorides in 32-56% overall yield and
Aldrichimica Acta, 28, 13-13 (1995)
Liu, C. et al.
Organic Process Research & Development, 1, 45-45 (1997)
C N Hodge et al.
Chemistry & biology, 3(4), 301-314 (1996-04-01)
Effective HIV protease inhibitors must combine potency towards wild-type and mutant variants of HIV with oral bioavailability such that drug levels in relevant tissues continuously exceed that required for inhibition of virus replication. Computer-aided design led to the discovery of
D Scholz et al.
Journal of medicinal chemistry, 37(19), 3079-3089 (1994-09-16)
A convenient procedure for the synthesis of 2-heterosubstituted statine derivatives as novel building blocks in HIV-protease inhibitors has been developed. The synthesis starts with protected L-phenylalaninols, which were converted to gamma-amino alpha, beta-unsaturated esters in a one-pot procedure. A highly
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系客户支持