所有图片(1)
About This Item
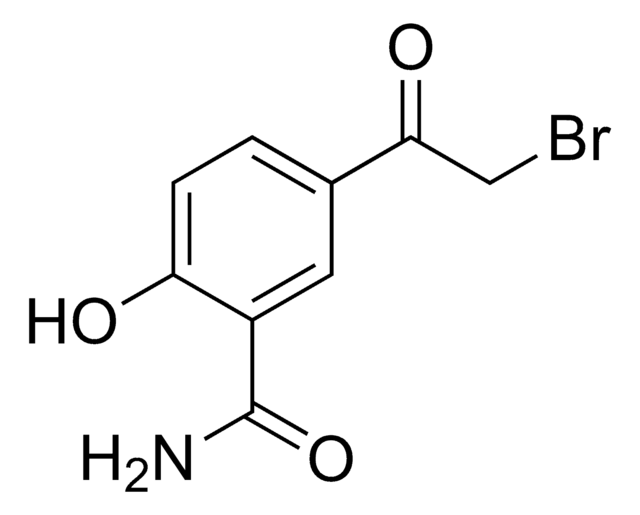
线性分子式:
CH3COC6H3(OH)CONH2
CAS号:
分子量:
179.17
EC號碼:
MDL號碼:
分類程式碼代碼:
12352100
PubChem物質ID:
推荐产品
化驗
98%
mp
220-222 °C (lit.)
SMILES 字串
CC(=O)c1ccc(O)c(c1)C(N)=O
InChI
1S/C9H9NO3/c1-5(11)6-2-3-8(12)7(4-6)9(10)13/h2-4,12H,1H3,(H2,10,13)
InChI 密鑰
LWAQTCWTCCNHJR-UHFFFAOYSA-N
一般說明
5-Acetylsalicylamide is formed by lewis acidic ionic liquid catalyzed Friedel-Crafts acylation of salicylamide with acetyl chloride.
應用
5-Acetylsalicylamide can be used in the preparation of 5-acetyl-2(3H)-benzoxazolone, via two-step synthetic route using Hofmann rearrangement.
訊號詞
Warning
危險分類
Acute Tox. 4 Dermal - Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
dust mask type N95 (US), Eyeshields, Gloves
Acylation of salicylamide to 5-acetylsalicylamide using ionic liquids as dual catalyst and solvent.
Chen W, et al.
Journal of Industrial and Engineering Chemistry (Amsterdam, Netherlands), 16(5), 800-804 (2010)
New heterocyclic chalcones. Part 6. Synthesis and cytotoxic activities of 5-or 6-(3-aryl-2-propenoyl)-2 (3H)-benzoxazolones.
Ivanova YB, et al.
Heterocyclic Communications, 19(1), 23-28 (2013)
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门