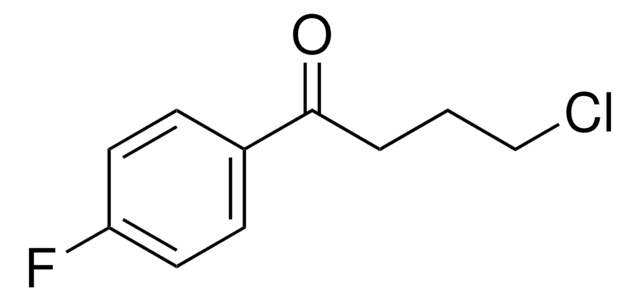
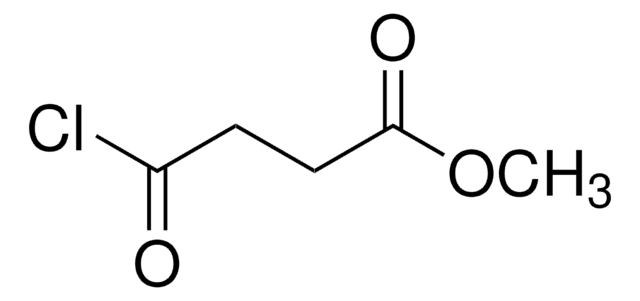
推荐产品
等級
technical grade
蒸汽壓力
3 mmHg ( 20 °C)
化驗
95%
形狀
liquid
自燃溫度
824 °F
折射率
n20/D 1.461 (lit.)
bp
173-174 °C (lit.)
密度
1.26 g/mL at 25 °C (lit.)
SMILES 字串
ClCCCC(Cl)=O
InChI
1S/C4H6Cl2O/c5-3-1-2-4(6)7/h1-3H2
InChI 密鑰
CDIIZULDSLKBKV-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
一般說明
4-Chlorobutyryl chloride is a chloroacyl chloride. It undergoes coupling with 4-carboxyphenylboronic acid under standard Suzuki coupling conditions [Pd(PPh3)4, Na2CO3, DME/H2O] to give 2′-methyl-4′-(2-oxo-1-pyrrolidinyl)biphenyl-4-carboxylic acid. Synthesis of 4-chlorobutyryl chloride using γ-butyroctone has been reported.3
應用
4-Chlorobutyryl chloride may be used in the radiosynthesis of (11)C-levetiracetam, a potential marker for PET imaging of SV2A expression. It may be used in the preparation of N-chloroacyl-6-O-triphenylmethylchitosans.
訊號詞
Danger
危險分類
Acute Tox. 2 Inhalation - Acute Tox. 4 Oral - Aquatic Acute 1 - Eye Dam. 1 - Met. Corr. 1 - Skin Corr. 1A
儲存類別代碼
6.1A - Combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
水污染物質分類(WGK)
WGK 1
閃點(°F)
185.0 °F
閃點(°C)
85 °C
個人防護裝備
Faceshields, Gloves, Goggles, type ABEK (EN14387) respirator filter
Multikilogram-scale synthesis of a biphenyl carboxylic acid derivative using a Pd/C-mediated suzuki coupling approach.
Ennis DS, et al.
Organic Process Research & Development, 3(4), 248-252 (1999)
Hancheng Cai et al.
ACS medicinal chemistry letters, 5(10), 1152-1155 (2014-10-15)
The multistep preparation of (11)C-levetiracetam ((11)C-LEV) was carried out by a one-pot radiosynthesis with 8.3 ± 1.6% (n = 8) radiochemical yield in 50 ± 5.0 min. Briefly, the propionaldehyde was converted to propan-1-imine in situ as labeling precursor by
Jukka Holappa et al.
Biomacromolecules, 6(2), 858-863 (2005-03-15)
An efficient synthetic route was developed for the mild chloroacylation of chitosan with different chloroacyl chlorides. Full N-chloroacylation was obtained with this procedure without any O-acylation, and products having lower degrees of substitution can also be produced. Organo-soluble 6-O-triphenylmethylchitosan was
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门