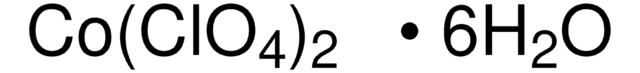所有图片(3)
About This Item
线性分子式:
CoBr2 · xH2O
CAS号:
分子量:
218.74 (anhydrous basis)
EC號碼:
MDL號碼:
分類程式碼代碼:
12161600
PubChem物質ID:
NACRES:
NA.22
推荐产品
形狀
solid
反應適用性
core: cobalt
reagent type: catalyst
SMILES 字串
O.Br[Co]Br
InChI
1S/2BrH.Co.H2O/h2*1H;;1H2/q;;+2;/p-2
InChI 密鑰
MOTZAWYGLXXRSO-UHFFFAOYSA-L
正在寻找类似产品? 访问 产品对比指南
應用
Catalyst for C-metal, C-heteroatom and C-C bond formations, addition to unsaturated bonds, cyclizations, functional group transformations, polymerization, oxidations and reductions, carbonylations.
訊號詞
Warning
危險分類
Acute Tox. 4 Dermal - Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
dust mask type N95 (US), Eyeshields, Gloves
Kei Murakami et al.
Organic letters, 11(11), 2373-2375 (2009-05-12)
Cobalt(II) bromide catalyzes arylzincation of alkynes with arylzinc iodide x lithium chloride complexes in acetonitrile. The scope of the arylzincation is wide enough to use unfunctionalized alkynes, such as 6-dodecyne, as well as arylacetylenes. The inherent functional group compatibility of
Efficient cobalt-catalyzed formation of unsymmetrical biaryl compounds and its application in the synthesis of a sartan intermediate.
Muriel Amatore et al.
Angewandte Chemie (International ed. in English), 47(11), 2089-2092 (2008-02-09)
Gerhard Hilt et al.
Chemical communications (Cambridge, England), (11), 1474-1475 (2005-03-10)
The intermolecular cyclotrimerisation of terminal and internal alkynes can be catalysed by simple cobalt complexes such as a CoBr2(diimine) under mild reaction conditions when treated with zinc and zinc iodide with high regioselectivity in excellent yields.
Hyacinthe Fillon et al.
Journal of the American Chemical Society, 125(13), 3867-3870 (2003-03-27)
A new chemical method for the preparation of arylzinc intermediates is described in acetonitrile, on the basis of the activation of aryl bromides by low-valent cobalt species arising from the reduction of cobalt halide by zinc dust. This procedure allows
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门