推荐产品
化驗
98%
形狀
solid
mp
75-77 °C (lit.)
溶解度
acetone: soluble 1%, clear, yellow
SMILES 字串
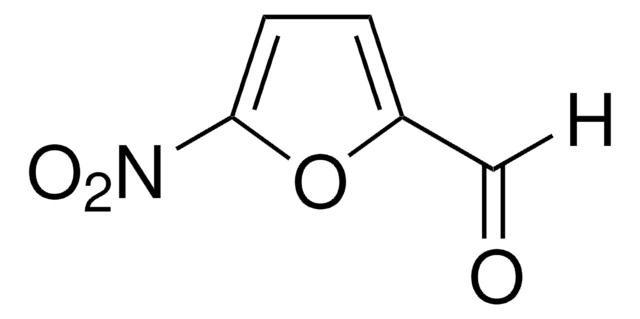
[H]C(=O)c1ccc(s1)[N+]([O-])=O
InChI
1S/C5H3NO3S/c7-3-4-1-2-5(10-4)6(8)9/h1-3H
InChI 密鑰
CHTSWZNXEKOLPM-UHFFFAOYSA-N
一般說明
Diastereoselectivity in [4+2] cycloaddition of 1-methoxy-2-methyl-3-(trimethylsiloxy)-1,3-pentadiene with 5-nitro-2-thiophenecarboxaldehyde was investigated.
應用
5-Nitro-2-thiophenecarboxaldehyde was used in preparation of 2, 3-dihydro-2-(5-nitro-2-thienyl) quinazolin-4-(1H)-ones and various novel oxime ether derivatives, anti-protozoan agents.
訊號詞
Warning
危險聲明
危險分類
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
dust mask type N95 (US), Eyeshields, Gloves
其他客户在看
Antibacterial 2,3-dihydro-2-(5-nitro-2-thienyl)-quinazolin-4(1H)-ones.
R J Alaimo et al.
Journal of medicinal chemistry, 15(3), 335-336 (1972-03-01)
Michael P Doyle et al.
Proceedings of the National Academy of Sciences of the United States of America, 101(15), 5391-5395 (2004-04-03)
Chiral dirhodium(II) carboxamidates are highly efficient catalysts for reactions between a variety of aldehydes and activated dienes. Catalyst loadings as low at 0.01 mol % have been realized with enantioselectivities up to 97%. Kinetic investigations reveal a pronounced electronic influence
Ali Almasirad et al.
Iranian journal of pharmaceutical research : IJPR, 10(4), 727-731 (2011-10-01)
A series of new 2-(phenylthio) benzoylarylhydrazones were synthesized by acid-catalyzed condensation of hydrazide 3 with corresponding aldehydes. The chemical structures of the compounds were elucidated by FT-IR, (1)H-NMR and Mass spectra. All newly synthesized compounds were evaluated for their antimycobacterial
Synthesis and< i> in vitro</i> anti-protozoan activity of new 5-nitrothiophene oxime ether derivatives.
Delmas F, et al.
European Journal of Medicinal Chemistry, 28(1), 23-27 (1993)
Jian Xu et al.
Food chemistry, 221, 1530-1538 (2016-12-17)
We synthesized a series of 4- or 5-functionalized TCT derivatives (1-12) and investigated their inhibitory activities and mechanisms on tyrosinase by using Spectrofluorimetry, 1H and 13C NMR titration and IR spectra. The results of the fluorescence spectra and NMR titrations
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门