推荐产品
產品線
ReagentPlus®
化驗
99%
形狀
solid
mp
232-234 °C (dec.) (lit.)
溶解度
95% ethanol: soluble 5%, clear to slightly hazy, light yellow to yellow
SMILES 字串
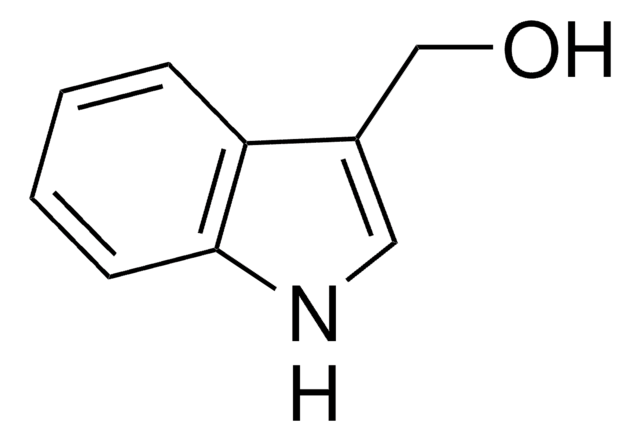
OC(=O)c1c[nH]c2ccccc12
InChI
1S/C9H7NO2/c11-9(12)7-5-10-8-4-2-1-3-6(7)8/h1-5,10H,(H,11,12)
InChI 密鑰
KMAKOBLIOCQGJP-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
一般說明
吲哚-3-羧酸衍生物的结构已采用高分辨质谱气相色谱(GC-HRMS)、超高效液相色谱结合高分辨率串联质谱(UHPLC-HRMS)、核磁共振光谱(NMR)和傅里叶变换红外光谱(FT-IR)进行了研究。
應用
作为反应物用于制备:
- 抗癌剂
- 氨基酸和肽的衍生物
- 5-羟色胺5-HT4受体拮抗剂
- 主要的酰基脲类
- 在Hedgehog途径中,Gli1介导的转录的抑制剂
- 5-羟色胺5-HT6拮抗剂
- 超晚期抗原-4(VLA-4)拮抗剂
- EphB3受体酪氨酸激酶抑制剂
- 阿尔茨海默病的潜在治疗药物
- 乙烯基酯假三肽蛋白酶体抑制剂
法律資訊
ReagentPlus is a registered trademark of Merck KGaA, Darmstadt, Germany
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
Eyeshields, Gloves, type N95 (US)
其他客户在看
Analytical characterization of some synthetic cannabinoids, derivatives of indole-3-carboxylic acid.
Vadim Shevyrin et al.
Forensic science international, 232(1-3), 1-10 (2013-09-24)
By means of gas chromatography with high resolution mass spectrometry (GC-HRMS), ultra-high performance liquid chromatography in combination with high resolution tandem mass spectrometry (UHPLC-HRMS), nuclear magnetic resonance spectroscopy (NMR) and Fourier transform infrared spectroscopy (FT-IR), structure of a series from
Xuejin Zhao et al.
Viruses, 13(8) (2021-08-29)
Influenza A viruses are serious zoonotic pathogens that continuously cause pandemics in several animal hosts, including birds, pigs, and humans. Indole derivatives containing an indole core framework have been extensively studied and developed to prevent and/or treat viral infection. This
J J Michnovicz et al.
Journal of the National Cancer Institute, 82(11), 947-949 (1990-06-06)
Dietary indoles in cruciferous vegetables induce cytochrome P450 enzymes and have prevented tumors in various animal models. Because estradiol metabolism is also cytochrome P450 mediated and linked to breast cancer risk, indoles may similarly reduce estrogen-responsive tumors in humans. We
Paweł Bednarek
Current opinion in plant biology, 15(4), 407-414 (2012-03-27)
In plants, a host's responses to an attempted infection include activation of various secondary metabolite pathways, some of which are specific for particular plant phylogenetic clades. Phytochemicals that represent respective end products in plant immunity have been stereotypically linked to
Mu-Yang Wang et al.
Journal of integrative plant biology, 54(7), 471-485 (2012-05-26)
Camalexin (3-thiazol-2'-yl-indole) is the major phytoalexin found in Arabidopsis thaliana. Several key intermediates and corresponding enzymes have been identified in camalexin biosynthesis through mutant screening and biochemical experiments. Camalexin is formed when indole-3-acetonitrile (IAN) is catalyzed by the cytochrome P450
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门