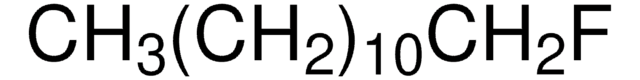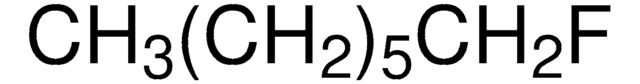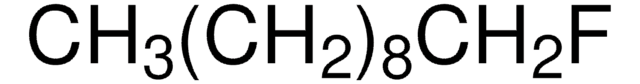推荐产品
质量水平
方案
98%
表单
liquid
折射率
n20/D 1.396 (lit.)
沸点
142-146 °C (lit.)
密度
0.814 g/mL at 25 °C (lit.)
官能团
alkyl halide
fluoro
SMILES字符串
CCCCCCCCF
InChI
1S/C8H17F/c1-2-3-4-5-6-7-8-9/h2-8H2,1H3
InChI key
DHIVLKMGKIZOHF-UHFFFAOYSA-N
一般描述
1-Fluorooctane undergoes C-F bond-cleavage reaction with phenyl magnesium chloride to give n-octylbenzene. It reacts rapidly with trimethylsilyl iodide to give corresponding octyl iodides and trimethylsilyl fluoride.
应用
1-Fluorooctane has been used as a molecular probe in evaluations of gas chromatographic stationary phases consisting of bromo- and chloro-derivatives of a C78 branched alkane.
警示用语:
Warning
危险分类
Eye Irrit. 2 - Flam. Liq. 3 - Skin Irrit. 2 - STOT SE 3
靶器官
Respiratory system
储存分类代码
3 - Flammable liquids
WGK
WGK 3
闪点(°F)
107.6 °F - closed cup
闪点(°C)
42 °C - closed cup
个人防护装备
Eyeshields, Gloves, type ABEK (EN14387) respirator filter
Halogen redistribution reactions between alkyl halides and trimethylsilyl iodide.
Friedrich EC and De Lucca G.
Journal of Organometallic Chemistry, 226(2), 143-148 (1982)
Kouki Matsubara et al.
Organic letters, 11(8), 1765-1768 (2009-03-14)
An unexpected C-F bond-cleavage reaction of unactivated fluoroalkanes with the well-known Grignard reagents without using metal catalysts has been discovered. For example, a reaction between 1-fluorooctane and phenyl magnesium chloride gave n-octylbenzene in moderate yield. This coupling reaction via the
Ervin Sz Kováts et al.
Journal of chromatography. A, 1113(1-2), 206-219 (2006-02-25)
In a paper published in 1992 [K.S. Reddy, J.-Cl. Dutoit, E.sz. Kováts, Pair-wise interactions by gas chromatography. I. Interaction free enthalpies of solutes with non-associated primary alcohol groups, J. Chromatogr. 609 (1992) 229] retention indices and standard chemical potential differences
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系客户支持