推荐产品
蒸汽密度
6.8 (vs air)
蒸汽壓力
2 mmHg ( 20 °C)
化驗
99%
形狀
liquid
包含
copper as stabilizer
折射率
n20/D 1.548 (lit.)
bp
170-172 °C (lit.)
密度
1.904 g/mL at 25 °C (lit.)
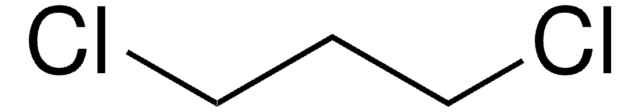
SMILES 字串
ClCCCI
InChI
1S/C3H6ClI/c4-2-1-3-5/h1-3H2
InChI 密鑰
SFOYQZYQTQDRIY-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
一般說明
1-氯-3-碘丙烷可与N-亚磺酰亚胺进行不对称的α-烷基化反应,生成2-取代的N-叔丁烷亚磺酰基-5-氯戊酰胺。通过循环伏安法对含有碳四正丁基铵的二甲基甲酰胺中玻璃碳电极上的1-氯-3-碘丙烷电还原反应进行了研究。它也参与到在水中将烷基碘共轭加成到α,β-不饱和腈上。
應用
1-氯-3-碘丙烷已被用于合成:
- N- [4- [5-(2,4-二氨基-6-氧代-1,6-二氢嘧啶-5-基)-2-(2,2,2-三氟乙酰基)戊基]苯甲酰基] -L-谷氨酸,一种甘氨酰胺核糖核苷酸转化酶(GAR Tfase)和氨基咪唑羧酰胺核糖核苷酸转化酶(AICAR Tfase)的抑制剂
- 有趣的″蛋白海绵″型分子喹[7,8-h]喹啉
訊號詞
Warning
危險聲明
危險分類
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
10 - Combustible liquids
水污染物質分類(WGK)
WGK 3
閃點(°F)
230.0 °F - closed cup
閃點(°C)
110 °C - closed cup
個人防護裝備
Eyeshields, Gloves, type ABEK (EN14387) respirator filter
其他客户在看
Synthesis of 1, 2, 3, 4-tetrahydroquinolines and 1, 2, 3, 4-tetrahydro-1, 6-naphthyridines by a directed lithiation reaction.
Reed JN, et al.
Tetrahedron Letters, 29(45), 5725-5728 (1988)
Fraser F Fleming et al.
The Journal of organic chemistry, 72(18), 6961-6969 (2007-08-10)
A new silica-supported zinc-copper matrix reagent promotes the conjugate addition of alkyl iodides to cyclic and acyclic alkenenitriles in water. X-ray diffraction and electron microscopy techniques suggest that the active copper species generated from elemental zinc and copper(I) iodide is
Electrochemical Reduction of 1, 3-Dihalopropanes at Carbon Cathodes in Dimethylformamide.
Pritts WA and Peters DG.
Journal of the Electrochemical Society, 141(4), 990-995 (1994)
Filip Colpaert et al.
The Journal of organic chemistry, 76(1), 234-244 (2010-12-02)
α-Alkylation of N-sulfinyl imidates with 1-chloro-3-iodopropane successfully led to 2-substituted N-tert-butanesulfinyl-5-chloropentanimidates in acceptable diastereomeric ratios (dr 67/33 to 72/28) and good yields (74-86%). Subsequent reduction with NaBH(4) led to the corresponding 2-substituted N-tert-butanesulfinyl-5-chloropentylamines, which could be cyclized to a range
Heng Cheng et al.
Bioorganic & medicinal chemistry, 13(10), 3593-3599 (2005-04-26)
The synthesis and evaluation of N-[4-[5-(2,4-diamino-6-oxo-1,6-dihydropyrimidin-5-yl)-2-(2,2,2-trifluoroacetyl)pentyl]benzoyl]-L-glutamic acid (2) as an inhibitor of glycinamide ribonucleotide transformylase (GAR Tfase) and aminoimidazole carboxamide ribonucleotide transformylase (AICAR Tfase) are reported. The inhibitor 2 was prepared in a convergent synthesis involving C-alkylation of methyl 4-(4,4,4-trifluoro-3-dimethylhydrazonobutyl)benzoate
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门