所有图片(2)
About This Item
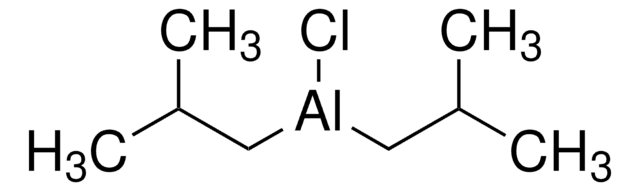
线性分子式:
[(CH3)2CHCH2]2AlH
CAS号:
分子量:
142.22
Beilstein:
4123663
MDL编号:
UNSPSC代码:
12352001
eCl@ss:
38120609
PubChem化学物质编号:
NACRES:
NA.22
推荐产品
表单
liquid
质量水平
反应适用性
reagent type: reductant
浓度
1.0 M in THF
沸点
65 °C
密度
0.866 g/mL at 25 °C
储存温度
2-8°C
SMILES字符串
CC(C)C[AlH]CC(C)C
InChI
1S/2C4H9.Al.H/c2*1-4(2)3;;/h2*4H,1H2,2-3H3;;
InChI key
AZWXAPCAJCYGIA-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
应用
二异丁基氢化铝溶液(1M;溶于THF中)是一种强力还原剂。它可用于以下反应:
- 合成经二肽保护的 反式-烯烃同构物。
- 原位生成双(1,5-环辛二烯)镍(0)(Ni(cod)2),其可催化将 乙烯基三丁基锡共轭加成至 2-丙烯醛,以形成 叔丁基二甲基[((E)-1,4-戊二烯基)氧基]硅烷。
- 还原芳基丙酸酯,以得到相应的炔丙醇。
用于Pd催化的仲烷基溴的还原脱溴过程。过苄基化呋喃糖苷的O-脱苄基和开环。方便从 ZrCp2Cl2 和DIBAL-H原位生成 HZrCp2Cl。
包装
查看可回收的容器产品。
警示用语:
Danger
危险分类
Acute Tox. 4 Oral - Carc. 2 - Eye Dam. 1 - Flam. Liq. 2 - Pyr. Liq. 1 - Skin Corr. 1B - STOT SE 3 - Water-react 1
靶器官
Central nervous system, Respiratory system
补充剂危害
储存分类代码
4.2 - Pyrophoric and self-heating hazardous materials
WGK
WGK 1
闪点(°F)
1.4 °F - closed cup
闪点(°C)
-17 °C - closed cup
个人防护装备
Faceshields, Gloves, Goggles
其他客户在看
A stereocontrolled synthesis of trans-alkene isosteres of dipeptides.
Spaltenstein A, et al.
Tetrahedron Letters, 27(19), 2095-2098 (1986)
Takashi Tomioka et al.
The Journal of organic chemistry, 76(11), 4669-4674 (2011-04-30)
Stepwise, selective DIBAL reduction of the acetonide diester derived from tartaric acid followed by the Horner-Emmons reaction effectively provided desymmetrized hydroxy mono-olefination products in a one-pot operation.
K Hirota et al.
Nucleic acids symposium series, (34)(34), 15-16 (1995-01-01)
It is reported that the diisobutylaluminum hydride (DIBALH) reduction of inosine and adenosine derivatives (1a and 1d) causes cleavage of the ribose moiety to give the corresponding 9-ribitylhypoxanthine (2a) and 9-ribityladenine (2d), respectively. The substitution effect of purine nucleosides on
Carl A Busacca et al.
The Journal of organic chemistry, 73(4), 1524-1531 (2008-01-17)
The reduction of tertiary phosphine oxides (TPOs) and sulfides with diisobutylaluminum hydride (DIBAL-H) has been studied in detail. An extensive solvent screen has revealed that hindered aliphatic ethers, such as MTBE, are optimum for this reaction at ambient temperature. Many
A direct entry to substituted N-methoxyamines from N-methoxyamides via N-oxyiminium ions.
Kenji Shirokane et al.
Angewandte Chemie (International ed. in English), 49(36), 6369-6372 (2010-07-31)
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系客户支持