推荐产品
蒸汽密度
2.35 (vs air)
品質等級
蒸汽壓力
1672 mmHg ( 55 °C)
31.66 psi ( 55 °C)
493 mmHg ( 20 °C)
9.22 psi ( 20 °C)
化驗
≥99%
形狀
liquid
包含
0.025 wt. % BHT as inhibitor
expl. lim.
14.3 %
折射率
n20/D 1.421 (lit.)
bp
32 °C/758 mmHg (lit.)
溶解度
alcohols: freely soluble
diethyl ether: freely soluble
water: insoluble
密度
0.936 g/mL at 25 °C (lit.)
運輸包裝
wet ice
儲存溫度
2-8°C
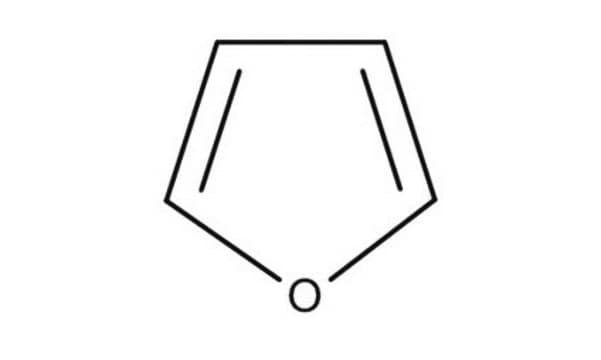
SMILES 字串
c1ccoc1
InChI
1S/C4H4O/c1-2-4-5-3-1/h1-4H
InChI 密鑰
YLQBMQCUIZJEEH-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
一般說明
應用
- Preparation of the starting material required for the synthesis of calix[6]pyrrole.
- To investigate the kinetics and mechanism of reactions of chlorine atoms with volatile organic compounds.
- Catalytic transformation of furan to aromatics and olefins.
訊號詞
Danger
危險分類
Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Aquatic Chronic 3 - Carc. 1B - Flam. Liq. 1 - Muta. 2 - Skin Irrit. 2 - STOT RE 2
安全危害
儲存類別代碼
3 - Flammable liquids
水污染物質分類(WGK)
WGK 3
閃點(°F)
-32.8 °F - closed cup
閃點(°C)
-36 °C - closed cup
個人防護裝備
Eyeshields, Faceshields, Gloves
其他客户在看
商品
The Diels–Alder reaction is the reaction between a conjugated diene and an alkene (dienophile) to form unsaturated six-membered rings. It is also referred to as a cycloaddition.
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门