推荐产品
化驗
97%
形狀
solid
mp
272-276 °C (lit.)
SMILES 字串
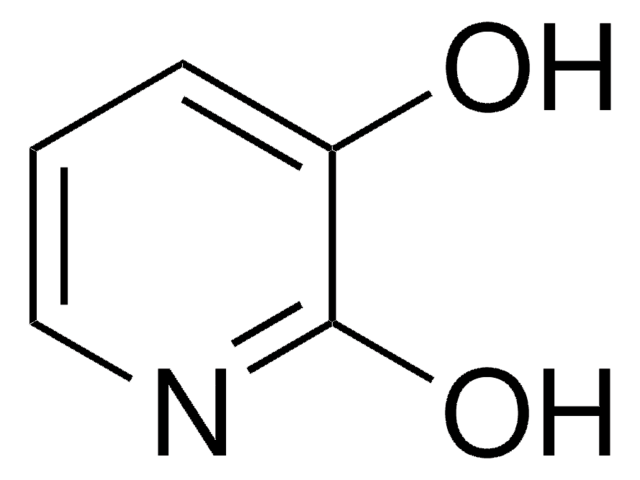
Oc1ccnc(O)c1
InChI
1S/C5H5NO2/c7-4-1-2-6-5(8)3-4/h1-3H,(H2,6,7,8)
InChI 密鑰
ZEZJPIDPVXJEME-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
一般說明
2,4-Dihydroxypyridine (3-deazauracil) is a potent inhibitor of dihydrouracil dehydrogenase.
應用
2,4-Dihydroxypyridine (3-deazauracil) was used in the synthesis of diazaphenoxathiin skeleton.
訊號詞
Warning
危險聲明
危險分類
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
dust mask type N95 (US), Eyeshields, Gloves
其他客户在看
J Molgó et al.
Journal de pharmacologie, 16 Suppl 2, 109-144 (1985-01-01)
In this review the effects of aminopyridines and chemically related compounds are documented in an attempt to analyse the mechanism underlying their presynaptic actions at the vertebrate neuromuscular junction. Aminopyridines and related compounds are of particular interest because they greatly
K T Lin et al.
Therapeutic drug monitoring, 5(4), 491-496 (1983-01-01)
A rapid and simple procedure for liquid chromatographic analysis of plasma 3-deazauridine (3-DU), an antineoplastic agent, was developed. The plasma was extracted with methanolic silver acetate to remove interfering ultraviolet-absorbing materials and the 3-DU partially purified on a small anion
K Fujita et al.
Drug metabolism and disposition: the biological fate of chemicals, 37(7), 1375-1377 (2009-04-25)
S-1 is an oral anticancer agent that combines tegafur, a prodrug of 5-fluorouracil (5-FU), and 5-chloro-2,4-dihydroxypyridine (CDHP), an inhibitor of dihydropyrimidine dehydrogenase. We examined the effects of aging on the pharmacokinetics of the components of S-1. The median area under
F P LaCreta et al.
Cancer research, 49(10), 2567-2573 (1989-05-15)
The breakdown of 5-fluoro-2'-deoxyuridine (FdUrd) to 5-fluorouracil (FUra) is catalyzed by the pyrimidine nucleoside phosphorylases, uridine phosphorylase and thymidine phosphorylase. The effects of nucleoside phosphorylase inhibitors on FdUrd and FUra elimination by the isolated perfused rat liver were investigated. The
M T Cocco et al.
European journal of medicinal chemistry, 35(5), 545-552 (2000-07-12)
4-hydroxy-2-pyridone derivatives 2 were prepared by reaction of 3-amino-3-dialkylaminopropenoates with bis(2,4, 6-trichlorophenyl)malonate. These compounds were further reacted with a set of aldehydes to give bis(pyridyl)methanes 3 and 4. The newly synthesized compounds 2, 3 and 4 were evaluated in vitro
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系客户支持