推荐产品
质量水平
方案
>98%
表单
solid
mp
72-74 °C (lit.)
储存温度
2-8°C
SMILES字符串
CC(=C)C(=O)Oc1ccc(cc1)C(C)(C)c2ccc(OC(=O)C(C)=C)cc2
InChI
1S/C23H24O4/c1-15(2)21(24)26-19-11-7-17(8-12-19)23(5,6)18-9-13-20(14-10-18)27-22(25)16(3)4/h7-14H,1,3H2,2,4-6H3
InChI key
QUZSUMLPWDHKCJ-UHFFFAOYSA-N
一般描述
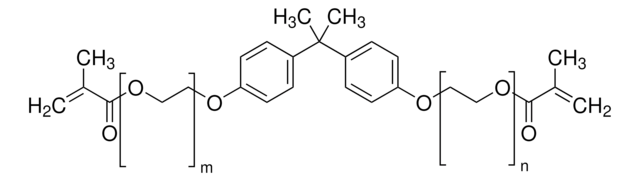
Bisphenol A dimethacrylate (BPA-DMA) is a key monomer, available in solid form with an assay of >98%, widely used in material science, particularly in the formulation of polymers and composite materials. BPA-DMA is primarily utilized as a crosslinking agent in the production of various thermosetting polymers. In dental applications, BPA-DMA is often incorporated into composites and adhesives due to its excellent bonding properties and ability to form durable, wear-resistant materials.
应用
Bisphenol A dimethacrylate (BPA-DMA) can be used as:
- A template in the synthesis of molecularly imprinted polymers (MIPs). The use of BPA-DMA allows for the formation of specific cavities in the polymer matrix that match the shape and functional groups of BPA, enhancing the selectivity and affinity of the resulting polymer for its target analyte.
- A co-monomer in the synthesis of photopolymerized monoliths for capillary electrochromatography.
- A key component in the production of bisphenol A-glycidyl methacrylate (Bis-GMA), which is widely used in dental restorative materials due to its mechanical properties and potential for additional functionalities like antibacterial activity.
警示用语:
Warning
危险声明
危险分类
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
靶器官
Respiratory system
储存分类代码
11 - Combustible Solids
WGK
WGK 3
闪点(°F)
Not applicable
闪点(°C)
Not applicable
个人防护装备
dust mask type N95 (US), Eyeshields, Gloves
其他客户在看
Stomatitis and perioral dermatitis caused by epoxy diacrylates in dental composite resins.
L Kanerva et al.
Journal of the American Academy of Dermatology, 38(1), 116-120 (1998-02-03)
R E Smith et al.
Biochimica et biophysica acta, 1550(1), 100-106 (2001-12-12)
The dental restorative monomer, BISGMA (2,2-bis[4-(2-hydroxy-3-methacryloxypropoxy)phenyl]propane), and bisphenol A diglycidyl ether (BADGE) increase the velocity of the reaction catalyzed by pancreatic cholesterol esterase (CEase, bovine). The metabolite of these monomers, bisphenol A bis(2,3-dihydroxypropyl) ether, and a common plasticizer, di-2-ethylhexyl phthalate
K Yoshida et al.
Dental materials : official publication of the Academy of Dental Materials, 8(2), 137-139 (1992-03-01)
Light-cured opaque resins were prepared using four types of monomer liquids and titanium dioxide powder. This study investigated the relationship between the monomer composition and the physical properties of light-cured opaque resin. Depth of cure, KHN, residual monomer eluent, and
Ravana Angelini Sfalcin et al.
Clinical oral investigations, 21(6), 2143-2151 (2016-11-14)
This study aimed at evaluating the chemophysical properties of experimental resin infiltrants (ERIs) doped with different bioactive particles. A control resin infiltrant (CR) was formulated using triethylene glycol dimethacrylate (TEGDMA) and ethoxylated bisphenol A dimethacrylate (BisEMA). Moreover, five experimental ERIs
Junjie Ou et al.
Journal of chromatography. A, 1217(22), 3628-3634 (2010-04-17)
A novel porous polymer monolith was prepared in situ in a fused-silica capillary using photoinitiated polymerization. Bisphenol A dimethacrylate (BPADMA) was selected as a crosslinker, copolymerized with benzyl methacrylate (BMA) in the presence of a binary porogenic solvent consisting of
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系客户支持