推荐产品
化驗
98%
形狀
solid
mp
55-58 °C (lit.)
官能基
chloro
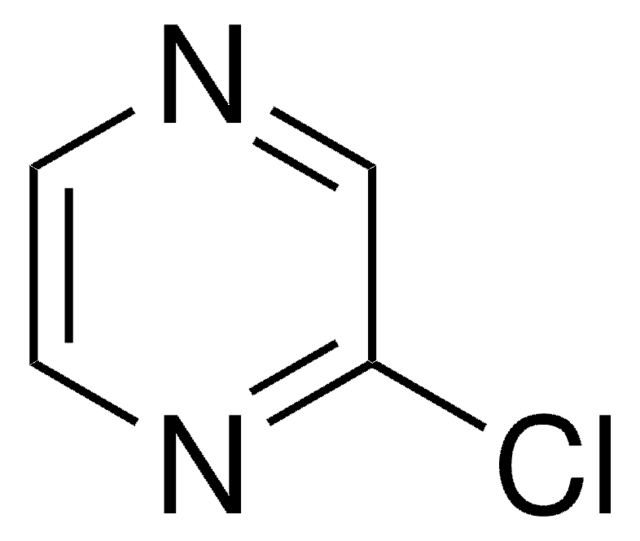
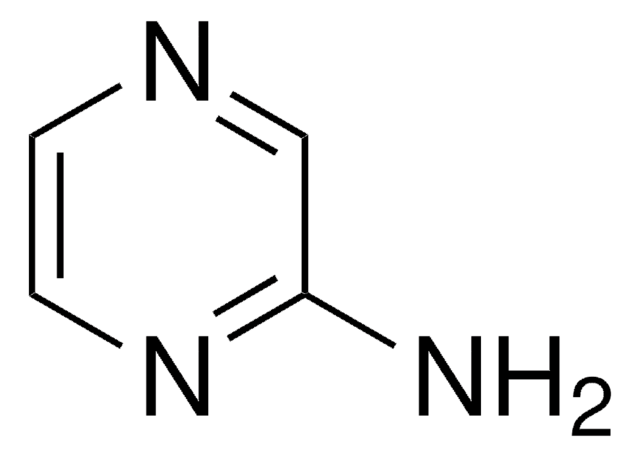
SMILES 字串
Clc1cncc(Cl)n1
InChI
1S/C4H2Cl2N2/c5-3-1-7-2-4(6)8-3/h1-2H
InChI 密鑰
LSEAAPGIZCDEEH-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
訊號詞
Warning
危險聲明
危險分類
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
235.4 °F - closed cup
閃點(°C)
113 °C - closed cup
個人防護裝備
dust mask type N95 (US), Eyeshields, Gloves
其他客户在看
Benjamin J Coe et al.
The Journal of organic chemistry, 75(24), 8550-8563 (2010-11-18)
Six new dicationic 2D nonlinear optical (NLO) chromophores with pyrazinyl-pyridinium electron acceptors have been synthesized by nucleophilic substitutions of 2,6-dichloropyrazine with pyridyl derivatives. These compounds have been characterized as their PF(6)(-) salts by using various techniques including electronic absorption spectroscopy
Laixing Hu et al.
Bioorganic & medicinal chemistry, 21(21), 6732-6741 (2013-09-10)
Dicationic 2,6-diphenylpyrazines, aza-analogues and prodrugs were synthesized; evaluated for DNA affinity, activity against Trypanosoma brucei rhodesiense (T. b. r.) and Plasmodium falciparum (P. f.) in vitro, efficacy in T. b. r. STIB900 acute and T. b. brucei GVR35 CNS mouse
Ling-Wei Kong et al.
Dalton transactions (Cambridge, England : 2003), 41(18), 5625-5633 (2012-03-17)
Cyclooligomerization of 2,6-dichloropyrazine 4 and benzyl 2,3-dihydroxybenzoate 5 under microwave irradiation resulted in a racemic pair of ester functionalized ortho-linked oxacalix[2]benzene[2]pyrazine 6, which was further transformed to the corresponding racemic carboxylic acid functionalized ortho-linked oxacalix[2]benzene[2]pyrazine 3. Both enantiomers of 3
Synthesis of oxacalixarenes incorporating nitrogen heterocycles: evidence for thermodynamic control.
Jeffrey L Katz et al.
Organic letters, 8(13), 2755-2758 (2006-06-16)
[structure: see text] Oxacalix[2]arene[2]hetarenes are formed in a single step by cyclooligomerization of meta-diphenols with meta-dichlorinated azaheterocycles. The high selectivity for cyclic tetramer formation results from thermodynamic product control. Macrocycles as large as oxacalix[5]arene[5]hetarenes have been isolated under nonequilibrating conditions.
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门