推荐产品
产品名称
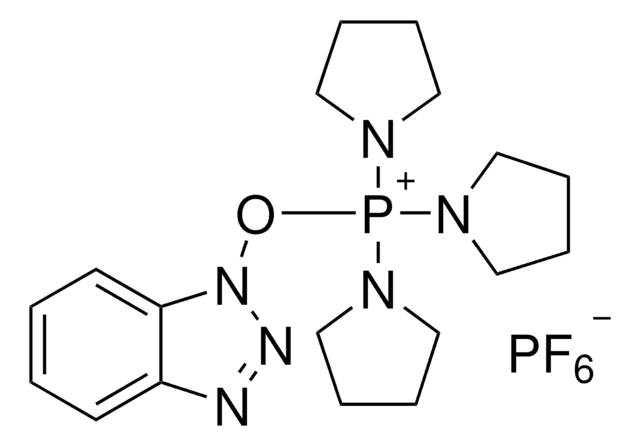
苯并三氮唑-N,N,N′,N′-四甲基脲六氟磷酸酯, ≥98.0% (T)
品質等級
化驗
≥98.0% (T)
形狀
solid
反應適用性
reaction type: Coupling Reactions
mp
200 °C (dec.) (lit.)
溶解度
acetonitrile: 0.1 g/mL, clear
應用
peptide synthesis
官能基
amine
儲存溫度
2-8°C
SMILES 字串
F[P-](F)(F)(F)(F)F.CN(C)C(\On1nnc2ccccc12)=[N+](/C)C
InChI
1S/C11H16N5O.F6P/c1-14(2)11(15(3)4)17-16-10-8-6-5-7-9(10)12-13-16;1-7(2,3,4,5)6/h5-8H,1-4H3;/q+1;-1
InChI 密鑰
UQYZFNUUOSSNKT-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
一般說明
HBTU是一种肽偶联剂,用于基于Fmoc的固相肽合成。
應用
HBTU是用于多肽合成的多肽偶联剂。它也被用于标准Fmoc固相多肽合成(SPPS)方案。
最近研究显示,这种偶联试剂的结晶或溶液结构是胍的 N-氧化物形式,,而不是脲化合物; 用于多肽合成的偶联试剂;优点是:极低的外消旋化,简单的反应条件,非常短的反应时间和高产率。
訊號詞
Warning
危險聲明
危險分類
Skin Sens. 1A
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
dust mask type N95 (US), Eyeshields, Gloves
其他客户在看
Hiroshi Wada et al.
PloS one, 13(7), e0199249-e0199249 (2018-07-04)
Recently, many autologous tumor antigens have been examined for their potential use in cancer immunotherapy. However, the success of cancer vaccines in clinical trials has been limited, partly because of the limitations of using single, short peptides in most attempts.
The uronium/guanidinium peptide coupling reagents: finally the true uronium salts.
Carpino LA, et al.
Angewandte Chemie (International Edition in English), 41(3), 441-445 (2002)
L P Miranda et al.
Proceedings of the National Academy of Sciences of the United States of America, 96(4), 1181-1186 (1999-02-17)
The chemical synthesis of peptides and small proteins is a powerful complementary strategy to recombinant protein overexpression and is widely used in structural biology, immunology, protein engineering, and biomedical research. Despite considerable improvements in the fidelity of peptide chain assembly
Héloise Boullet et al.
Molecules (Basel, Switzerland), 24(9) (2019-05-06)
Antimicrobial peptides (AMPs) are considered as potential therapeutic sources of future antibiotics because of their broad-spectrum activities and alternative mechanisms of action compared to conventional antibiotics. Although AMPs present considerable advantages over conventional antibiotics, their clinical and commercial development still
Mark Lommel et al.
Scientific reports, 8(1), 11753-11753 (2018-08-08)
Thrombospondins (TSPs) are multidomain glycoproteins with complex matricellular functions in tissue homeostasis and remodeling. We describe a novel role of TSP as a Wnt signaling target in the basal eumetazoan Hydra. Proteome analysis identified Hydra magnipapillata TSP (HmTSP) as a
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门